ESMO 2019: Invited Discussant: The CARD Trial - Cabazitaxel as Third Line Therapy in Metastatic Castration-Resistant Prostate Cancer
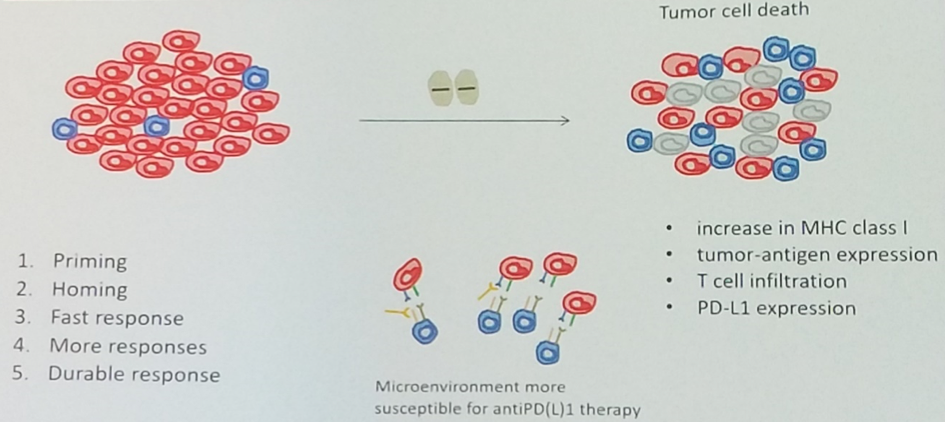
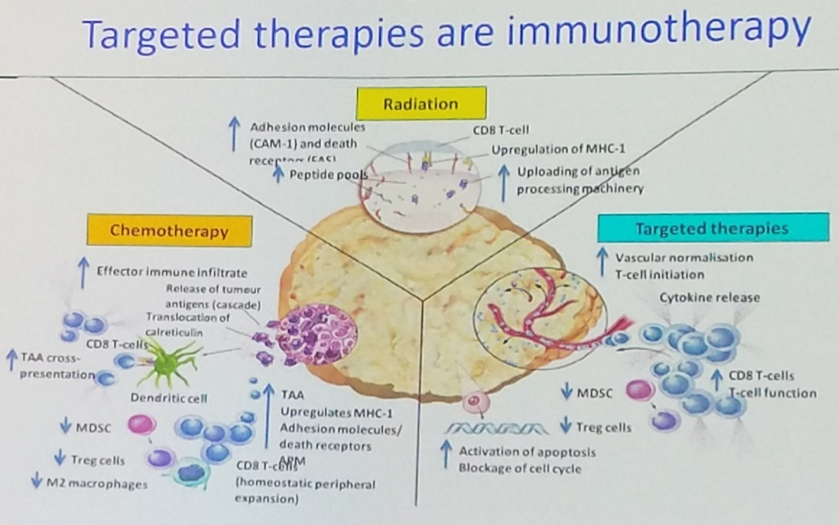
Barcelona, Spain (UroToday.com) In this discussion of the CARD trial of cabazitaxel as third line therapy in metastatic castration-resistant prostate cancer (mCRPC), Silke Gillessen, MD, began by laying out the therapeutic landscape of mCRPC, using this imagery to highlight what happens after up-front anti-androgen therapy and docetaxel chemotherapy, because it is less clear what the third-line treatment of choice should be.

The CARD study answers this question, showing that the cabazitaxel as third-line therapy is superior with regards to radiographic progression free survival (PFS) in patients who relatively rapidly progress through first-line anti-androgen therapy. This supports the idea that patients who do not have extended responses to anti-androgen therapy need cytotoxic therapy rather than another anti-androgen agent.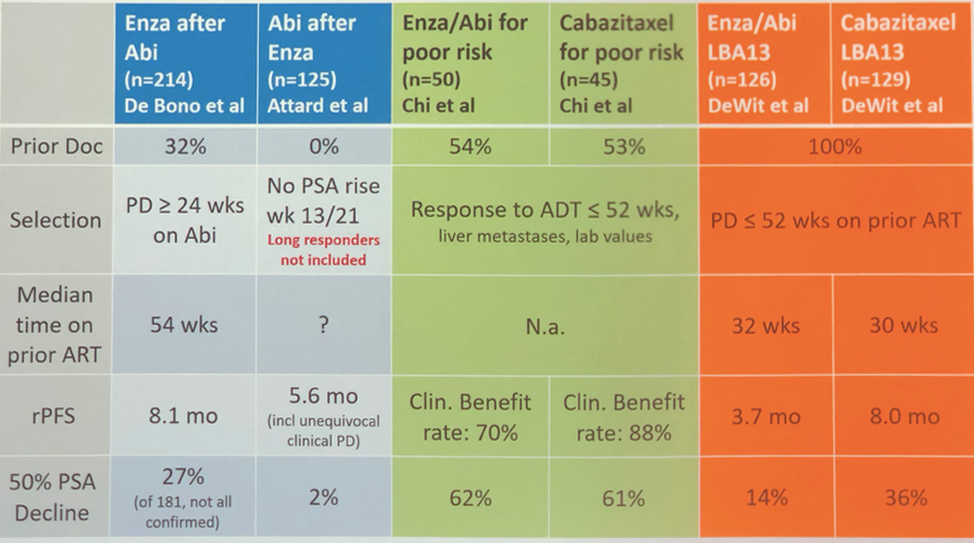
Though many providers and patients may have chemophobia, toxicity in the cabazitaxel arm was not markedly worse than the control arm.
There are a few important points to note. Though the PROSELICA trial showed equivalency between 20 mg/m2 and 25 mg/m2 of cabazitaxel, the CARD protocol used the 25 mg/m2 dose and G-CSF prophylaxis, with only a few cases of febrile neutropenia. There is no indication that the US FDA approved dose would be any less effective as to third line therapy. Second, these data do not answer the question of appropriate third line therapy in patients who respond to anti-androgen therapy for longer than a year. Could these patients uniquely benefit from subsequent anti-androgen therapy? Third, could cabazitaxel be moved forward into the castration-sensitive stage based on this data, and would patient outcomes change if it was given prior to docetaxel?
While further study could help understand these lingering questions, Dr. Gillessen believes that this well designed clinical trial clearly identifies cabazitaxel as the third-line treatment of choice in patients who progressed on anti-androgen therapy within 12 months. In light of the PROfound data showing the efficacy of olaparib as a third line therapy in patients with selected DNA damage repair gene defects, further work and discussion is required to understand how to best approach third line therapy in chemofit patients who have these genetic defects and have progressed through anti-androgen therapy in less than 12 months.
Presented by: Silke Gillessen, MD, senior consultant in the Medical Oncology-Hematology Department at the Kantonsspital St. Gallen, St Gallen, Switzerland and Co-founder of the Advanced Prostate Cancer Consensus Conference (APCCC), previously of the University of Manchester and the NHS Christie Trust, Manchester, United Kingdom
Written by: Alok Tewari, MD, PhD, Medical Oncology Fellow at the Dana-Farber Cancer Institute, at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept - 1 Oct 2019 in Barcelona, Spain