Non-muscle-invasive bladder Cancer (NMIBC) is a diverse condition featuring varying progression and relapse rates which are influenced by several clinical and pathological factors.5-8 Intravesical Bacillus Calmette-Guérin (BCG) therapy is widely acknowledged as effective against this condition, and this is the reason why it was the initial treatment adopted to reduce the risk of relapse and progression in high-grade NMIBC cases.5-8 Approximately 70% of patients can achieve complete remission depending on their risk profile. However, up to 60% of them may experience relapse within a year.8,9 Approximately 20% of high-risk cases may progress to muscle-invasive disease within four years despite the BCG treatment application.8-10
A critical aspect to be taken into consideration in cases of NMIBC relapse, despite adequate BCG therapy application, lies in whether there is a large enough window of time to explore alternative treatment options before contemplating radical cystectomy. Evidence available in the literature has suggested that most patients have approximately one year before experiencing prognosis decline.11 Moreover, a study did not find significant differences in five-year overall or cancer-specific survival rates between patients participating in clinical trials and those who chose the option for cystectomy right away.12 However, it is noteworthy that patients presenting any lymphovascular invasion or prostatic urethral involvement degree tend to have poorer prognosis and may not be suitable candidates for second-line therapy.
In cases where intravesical treatment options proved to be ineffective, the recommended course of action is radical cystectomy with intestinal diversion. While radical cystectomy has the potential to halt local disease progression, it is accompanied by a substantial risk of postoperative complications, diminished quality of life for patients, and the potential for overtreatment of individuals whose disease may not be significantly advanced. Furthermore, not all patients are suitable candidates for cystectomy due to factors such as advanced age or the presence of severe comorbidities. Considering the life-altering consequences and the adverse impact on quality of life associated with radical cystectomy, a significant number of patients opt to decline this surgical procedure. Consequently, there exists an urgent need for the development of innovative therapies that can offer a non-surgical second-line treatment option.
The BCG non-response point is a crucial time for patients. It is well-established that a third BCG therapy course provides minimal benefit and means the option for undergoing aggressive second-line therapy over cystectomy can potentially lead to tumor progression, among other unfavorable outcomes. On the other hand, radical cystectomy is associated with increased comorbidity rates and may represent over-treatment. Therefore, it is imperative to engage in comprehensive and careful discussions with patients before making these life-changing decisions.
In addition to challenges related to BCG’s toxicity and therapeutic ineffectiveness in high-grade NMIBC patients, the ongoing global shortage in BCG production, as well as its distribution disruptions, contribute to significantly worsening patients’ prognoses. The current BCG shortage has forced clinics to implement rationing and prioritization strategies, and it may have contributed to increased disease relapse and progression rates among NMIBC patients.13-15 Given the aforementioned BCG shortage, patients have been subjected to reduced BCG therapy courses, a fact that may increase the number of patients undergoing cystectomy.13 Moreover, the BCG shortage has also affected patients’ access and eligibility to participate in clinical trials.13
Nowadays, second-line treatment options available for BCG-unresponsive NMIBC cases are less than ideal. Although several conventional chemotherapeutic agents, such as gemcitabine, mitomycin, gemcitabine combined with mitomycin, docetaxel, and valrubicin, have been explored, none of them was superior to BCG and remains under investigation.
The bladder cancer treatment scenario has significantly evolved, since it not only encompasses traditional approaches, such as surgery and chemotherapy, but also immunotherapy integration. Modulating the tumor microenvironment (TME) and enhancing T cell infiltration into tumors are pivotal strategies to assist in managing different tumor types.16 One of the effective approaches involves using innate immunity modulators, more specifically, toll-like receptor (TLR) agonists.16,17 These agonists aid in increasing the activity of anti-tumor effector cells, such as cytotoxic T cells and natural killer cells, while simultaneously suppressing immunosuppressive cell populations, such as regulatory T cells (Tregs) and myeloid suppressor cells. Furthermore, they play a significant role in influencing immune cells’ recruitment within the tumor microenvironment.16,17 The emergence of novel immunotherapeutic agents has revolutionized the treatment applied to different tumor types, such as NMIBC.5
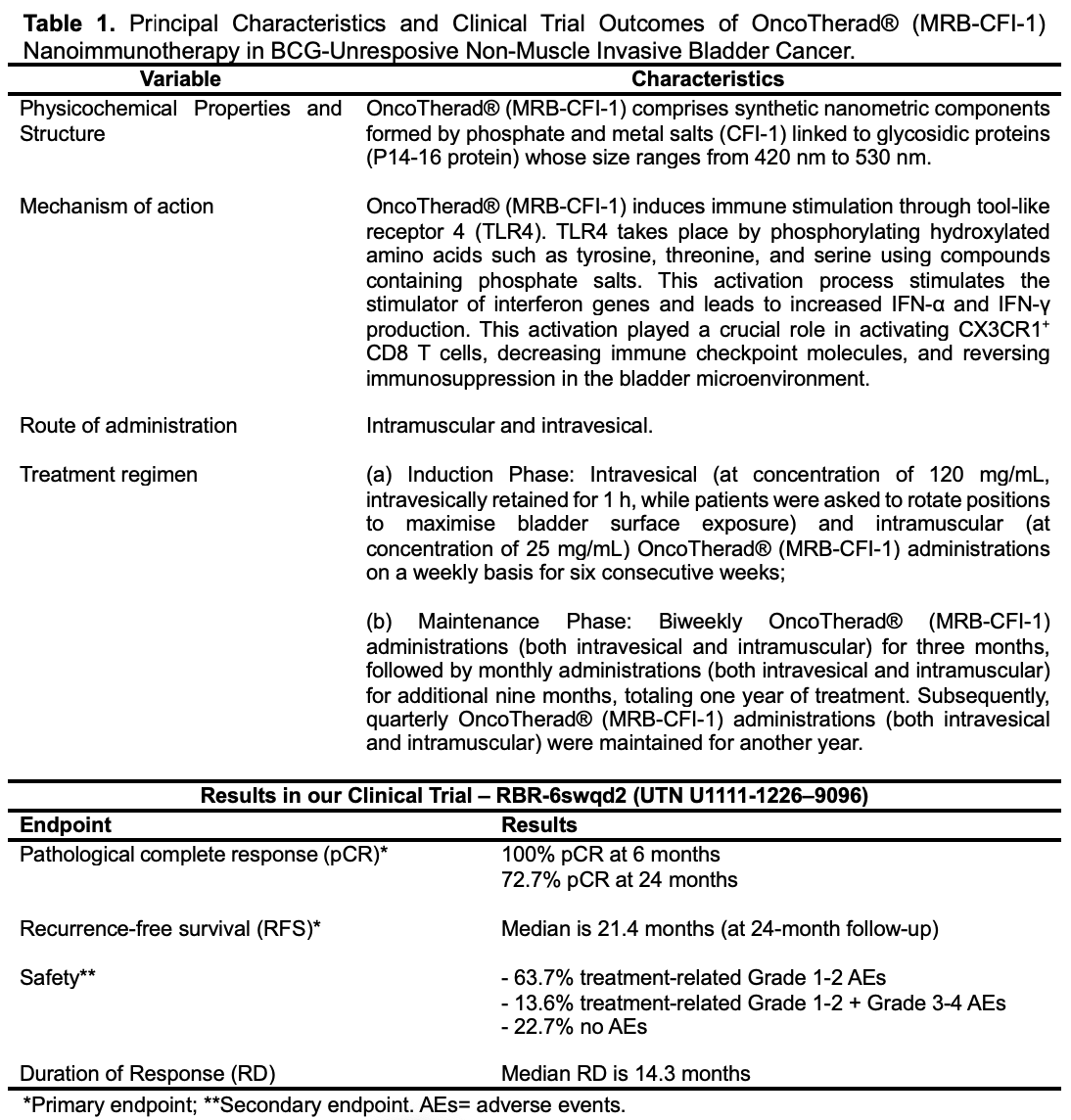
Accordingly, our research team has developed the OncoTherad® (MRB-CFI-1) nanoimmunotherapy, which recorded favorable outcomes in cancer therapy, mainly in NMIBC cases.18-26 OncoTherad® (MRB-CFI-1) comprises nanometric components formed by phosphate and metal salts (CFI-1) linked to glycosidic proteins (P14 and P16 proteins) whose size ranges from 420 nm to 530 nm.18 It holds granted patents both in the United States (USPTO: US-11623869-B2; US-11572284-B2; US-11136242-B2; US-11639294-B2) and in Brazil (INPI: BR102017012768B1).20-23
OncoTherad® (MRB-CFI-1) triggers the human innate immune system by activating TLR2 and TLR4. This process enhances the IFN signaling pathway activation process, which involves TLR4, TRIF, IRF-3, IFN-α, and IFN-γ.18,19,24-26 More specifically, OncoTherad® (MRB-CFI-1) induces immune stimulation through TLR4. TLR4 takes place by phosphorylating hydroxylated amino acids such as tyrosine, threonine, and serine using compounds containing phosphate salts. This activation process stimulates the stimulator of interferon genes (STING) and leads to increased IFN-α and IFN-γ production.18,19,24-26 Moreover, OncoTherad® (MRB-CFI-1) downregulates the expression of both the receptor activator of nuclear factor-κB (RANK) and the receptor activator of the nuclear factor-κB ligand (RANK-L) system. Therefore, it aids in preventing metastasis formation and inhibiting disease progression.18,19,24-26
In our study, we employed assessed the safety and efficacy of OncoTherad® (MRB-CFI-1) nanoimmunotherapy for NMIBC patients unresponsive to BCG and explored its mechanisms of action in a bladder cancer microenvironment26. A single-arm phase I/II study (Clinical Trial: RBR-6swqd2; UTN U1111-1226–9096), single-center (Municipal Hospital of Paulinia, São Paulo, Brazil) was conducted with 44 patients (30 male, 14 female). Primary outcomes were pathological complete response (pCR) and relapse-free survival (RFS). Secondary outcomes comprised response duration (RD) and therapy safety. Patients’ mean age was 65 years; 59.1% of them were refractory, 31.8% relapsed, and 9.1% were intolerant to BCG. OncoTherad® (MRB-CFI-1) nanoimmunotherapy showed pCR rate (95% CI) after 6 months of 100% (95% CI) and 72.7% (12/44) after 24 months. The Kaplan–Meier curve plotted for RFS indicated a mean RFS of 21.4 months (equivalent to 642 days) during the 24-month follow-up of patients treated with OncoTherad® (MRB-CFI-1). Moreover, the mean RD was 14.3 months.
Concerning the safety of OncoTherad® (MRB-CFI-1) nanoimmunotherapy, thirty-four (77.3%) of the herein assessed 44-patient cohort experienced adverse reactions attributable to treatment, whereas 10 patients (22.7%) remained free from any discernible adverse effects. Grade 1–2 adverse reactions were significantly more prevalent than the grade 3–4 ones. More specifically, 28 patients (63.7%) exclusively presented grade 1–2 adverse reactions, whereas six patients (13.6%) experienced both grade 1–2 and 3–4 adverse reactions. Dysuria, itching, cystitis, arthralgia, fatigue, skin rash, and fever were the grade 1–2 adverse reactions (≥20% incidence) most reported by patients. Conversely, the most common grade 3–4 adverse reactions (≥4% incidence) comprised skin rash, diarrhea, cough, and shortness of breath. Furthermore, comprehensive biochemical serological analyses did not show significant differences in parameters such as glucose, hemoglobin, leukocyte count, and platelet count, as well as AST, ALT, GGT, urea, and creatinine levels, before and after OncoTherad® (MRB-CFI-1) treatment application.
In relation to the mechanisms of action of this nanoimmunotherapy, OncoTherad® (MRB-CFI-1) activated the innate immune system through toll-like receptor 4, leading to increased interferon signaling. This activation played a crucial role in activating CX3CR1+ CD8 T cells, decreasing immune checkpoint molecules, and reversing immunosuppression in the bladder microenvironment.
OncoTherad® (MRB-CFI-1) nanoimmunotherapy emerges as a safe and effective treatment option for patients with BCG-unresponsive NMIBC and for those who have undergone intravesical chemotherapy. This therapy has the potential to help reduce tumor relapse rates and potentially delay or rule out the need for conducting radical surgical interventions in these patients.
Our study has certain limitations that should be acknowledged. Firstly, the relatively small sample size may not provide a precise reflection of the safety and efficacy of OncoTherad® (MRB-CFI-1) immunotherapy. It is important to note that our results are derived from a single institution with expertise in NMIBC treatment protocols. However, it is conceivable that these findings may not be readily applicable to other institutions with lower case volumes of NMIBC.
Moreover, our single-arm study was constrained by the absence of a direct comparator group. Nevertheless, it’s worth emphasizing that there are limited effective treatment alternatives available beyond radical cystectomy for this patient population. Additionally, comparing our findings with off-label treatments is a complex endeavor due to historical inconsistencies in defining adequate BCG therapy, variations in BCG dosing regimens, the inherent heterogeneity of patient populations, and differences in monitoring or surveillance protocols.
In recognition of these limitations, our research group is actively pursuing further investigations to substantiate the safety and efficacy of OncoTherad® (MRB-CFI-1) immunotherapy. Our future research initiatives will encompass expansions to multiple clinical research centers and a substantial augmentation of the sample size.
Written by: João Carlos Cardoso Alonso, PhD1,2 and Wagner José Fávaro, PhD1
- Laboratory of Urogenital Carcinogenesis and Immunotherapy (LCURGIN), Universidade Estadual de Campinas (UNICAMP), Campinas 13083-865, São Paulo, Brazil.
- Paulínia Municipal Hospital, Paulínia 13140-000, São Paulo, Brazil
- Faguet, G.B. A brief history of cancer: Age-old milestones underlying our current knowledge database. Int. J. Cancer 2015, 136, 2022–2036.
- Celada Luis, G.; Albers Acosta, E.; de la Fuente, H.; Velasco Balanza, C.; Arroyo Correas, M.; Romero-Laorden, N.; Alfranca, A.; Olivier Gómez, C. A Comprehensive Analysis of Immune Response in Patients with Non-Muscle-Invasive Bladder Cancer. Cancers 2023, 15, 1364.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Allard, P.; Bernard, P.; Fradet, Y.; Têtu, B. The early clinical course of primary Ta and T1 bladder cancer: A proposed prognostic index. Br. J. Urol. 1998, 81, 692–698.
- Lobo, N.; Martini, A.; Kamat, A.M. Evolution of immunotherapy in the treatment of non-muscle-invasive bladder cancer. Expert Rev. Anticancer Ther. 2022, 22, 361–370.
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657.
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354.
- Shore, N.D.; Palou Redorta, J.; Robert, G.; Hutson, T.E.; Cesari, R.; Hariharan, S.; Rodríguez Faba, Ó.; Briganti, A.; Steinberg, G.D. Non-muscle-invasive bladder cancer: An overview of potential new treatment options. Urol. Oncol. 2021, 39, 642–663.
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck,W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–477.
- Van den Bosch, S.; Witjes, A.J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: A systematic review. Eur. Urol. 2011, 60, 493–500.
- Jäger, W.; Thomas, C.; Haag, S.; Hampel, C.; Salzer, A.; Thuroff, J.W.; Wiesner, C. Early vs. delayed radical cystectomy for ‘high-risk’ carcinoma not invading bladder muscle: Delay of cystectomy reduces cancer-specific survival. BJU Int. 2011, 108, E284–E288.
- Haas, C.R.; Barlow, L.J.; Badalato, G.M.; De Castro, G.J.; Benson, M.C.; McKiernan, J.M. The timing of radical cystectomy for bacillus Calmette-Guérin failure: Comparison of outcomes and risk factors for prognosis. J. Urol. 2016, 195, 1704–1709.
- Guallar-Garrido, S.; Julián, E. Bacillus Calmette-Guérin (BCG) Therapy for Bladder Cancer: An Update. Immunotargets Ther. 2020, 9, 1–11.
- Grimm, M.O.; van der Heijden, A.G.; Colombel, M.; Muilwijk, T.; Martínez-Piñeiro, L.; Babjuk, M.M.; Türkeri, L.N.; Palou, J.; Patel, A.; Bjartell, A.S.; et al. Treatment of High-Grade Non-Muscle-Invasive Bladder Carcinoma by Standard Number and Dose of BCG Instillations versus Reduced Number and Standard Dose of BCG Instillations: Results of the European Association of Urology Research Foundation Randomised Phase III Clinical Trial “NIMBUS”. Eur. Urol. 2020, 78, 690–698.
- Ourfali, S.; Ohannessian, R.; Fassi-Fehri, H.; Pages, A.; Badet, L.; Colombel, M. Recurrence Rate and Cost Consequence of the Shortage of Bacillus Calmette-Guérin Connaught Strain for Bladder Cancer Patients. Eur. Urol. Focus 2021, 7, 111–116.
- Yang, Y.; Feng, R.; Wang, Y.Z.; Sun, H.W.; Zou, Q.M.; Li, H.B. Toll-like receptors: Triggers of regulated cell death and promising targets for cancer therapy. Immunol. Lett. 2020, 223, 1–9.
- Bourquin, C.; Pommier, A.; Hotz, C. Harnessing the immune system to fight cancer with Toll-like receptor and RIG-I-like receptor agonists. Pharmacol. Res. 2020, 154, 104192.
- Fávaro,W.J.; Alonso, J.C.C.; de Souza, B.R.; Reis, I.B.; Gonçalves, J.M.; Deckmann, A.C.; Oliveira, G.; Dias, Q.C.; Durán, N. New synthetic nano-immunotherapy (OncoTherad®) for non-muscle invasive bladder cancer: Its synthesis, characterization and anticancer property. Tissue Cell 2023, 80, 101988.
- Reis, I.B.; Tibo, L.H.S.; de Souza, B.R.; Durán, N.; Fávaro, W.J. OncoTherad® is an immunomodulator of biological response that downregulate RANK/RANKL signaling pathway and PD-1/PD-L1 immune checkpoint in non-muscle invasive bladder cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 5025–5036.
- Fávaro,W.J.; Durán-Caballero, N.E. Method for Producing a Nanostructured Complex (CFI-1), a Protein-Associated Nanostructured Complex (MRB-CFI-1) and Use. U.S. Patent 16/617,493, 10 May 2021.
- Fávaro,W.J.; Durán-Caballero, N.E. Method for Producing a Nanostructured Complex (CFI-1), a Protein-Associated Nanostructured Complex (MRB-CFI-1) and Use. U.S. Patent 17/236,839, 11 April 2023.
- Fávaro,W.J.; Durán-Caballero, N.E. Method for Producing a Nanostructured Complex (CFI-1), a Protein-Associated Nanostructured Complex (MRB-CFI-1) and Use. U.S. Patent 17/236,848, 2 July 2023.
- Fávaro,W.J.; Durán-Caballero, N.E. Method for Producing a Nanostructured Complex (CFI-1), a Protein-Associated Nanostructured Complex (MRB-CFI-1) and Use. U.S. Patent 17/236,861, 5 February 2023.
- Reis, S.K.; Socca, E.A.R.; de Souza, B.R.; Genaro, S.C.; Durán, N.; Fávaro,W.J. Effects of combined OncoTherad immunotherapy and probiotic supplementation on modulating the chronic inflammatory process in colorectal carcinogenesis. Tissue Cell 2022, 75, 101747.
- Ribeiro de Souza, B.; Brum Reis, I.; Cardoso de Arruda Camargo, G.; Oliveira, G.; Cristina Dias, Q.; Durán, N.; José Fávaro, W. A novel therapeutic strategy for non-muscle invasive bladder cancer: OncoTherad® immunotherapy associated with platelet-rich plasma. Int. Immunopharmacol. 2023, 123, 110723.
- Alonso, J.C.C.; de Souza, B.R.; Reis IB, de Arruda Camargo, G.C.; de Oliveira, G.; de Barros Frazão Salmazo, M.I.; Gonçalves, J.M.; de Castro Roston, J.R.; Caria, P.H.F.; da Silva Santos, A.; de Freitas, L.L.L.; Billis, A.; Durán, N.; Fávaro W.J. OncoTherad® (MRB-CFI-1) Nanoimmunotherapy: A Promising Strategy to Treat Bacillus Calmette-Guérin-Unresponsive Non-Muscle-Invasive Bladder Cancer: Crosstalk among T-Cell CX3CR1, Immune Checkpoints, and the Toll-Like Receptor 4 Signaling Pathway. Int J Mol Sci, 24, 17535.


