Introduction
Bacillus Calmette Guerin (BCG) is currently guideline-recommended in the adjuvant setting for patients with intermediate or high-risk non-muscle invasive bladder cancer (NMIBC).1 This is based on the results of numerous randomized clinical trials and meta-analyses demonstrating its ability to reduce the rates of disease recurrence and progression, compared to transurethral resection of bladder tumor (TURBT) alone or other adjuvant therapies.2-5
However, long-term results of a phase 3 trial in this setting demonstrate that the risk of disease recurrence remains approximately 40%, with ≥10% of patients experiencing progression to muscle-invasive disease.2 As such, there has been increased interest in combining BCG with other agents in this disease space to further improve on these recurrence and progression rates. However, given the ongoing worldwide BCG shortage, alternatives to BCG for patients with high risk NMIBC have also emerged as another unmet clinical need.
This Center of Excellence article will summarize the current and emerging evidence for intravesical chemotherapy, either alone or in combination with BCG, for patients with BCG-naïve NMIBC.
BCG Combinations
Mitomycin + BCGIt has been hypothesized that BCG-induced inflammation might increase the permeability of the bladder mucosa, enhancing the ability of intravesical mitomycin and other agents to reach target cancer tissue more easily and subsequently enhance their efficacy. In return, mitomycin C induces immunogenic cell death, enhancing the expression of specific damage signals, like HMGB1 molecule, that subsequently favors the phagocytosis of dying tumor cells, the activation of innate immune cells, and the presentation of tumor antigens to T lymphocytes.6
Di Stasi et al. conducted an RCT comparing BCG alone (n=105) to sequential BCG + electromotive mitomycin (n=107) in patients with stage T1 bladder cancer. In the sequential treatment arm, patients received 81 mg BCG for once a week over two weeks, followed by electromotive mitomycin once a week for 3 weeks. Conversely, patients in the BCG alone arm received 6 weeks of once weekly BCG, and complete responders in either arm subsequently underwent maintenance treatment. At a median follow-up of 88 months, patients in the sequential BCG plus electromotive mitomycin had a significantly longer disease-free interval (69 versus 21 months, p=0.0012):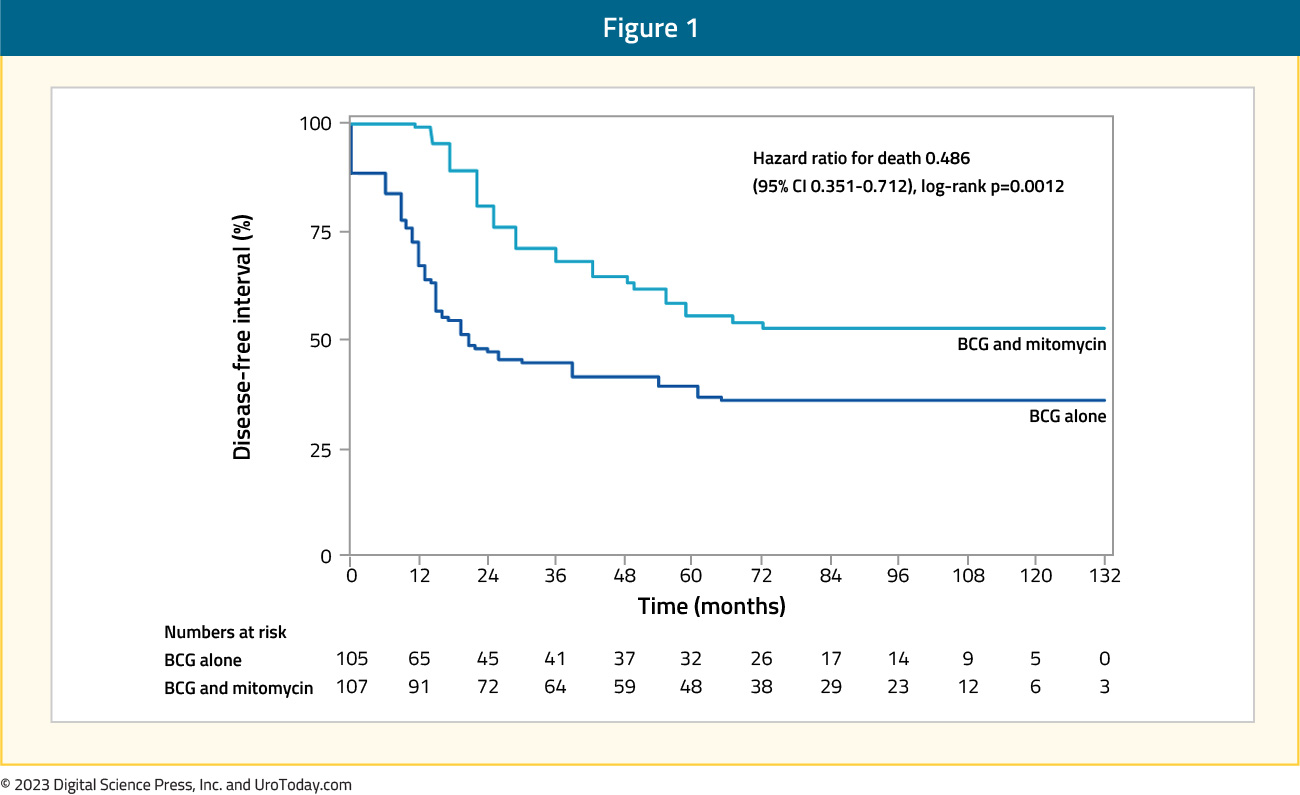
as well as lower risks of disease recurrence (42% versus 58%), progression (9% versus 22%), and cancer-specific deaths (6% versus 16%). The majority of the adverse effects were local in nature, and there were no significant group differences in the frequency or severity of side effects.7
In 2015, the final results of CUETO 93009 were published in European Urology. This RCT randomized 407 patients with intermediate- to high-risk NMIBC to either BCG + mitomycin C (n=211) or BCG alone (n=196). Patients in the sequential combination arm of mitomycin + BCG had a significantly improved 5-year disease-free interval (HR 0.57, 95% CI 0.39 to 0.83), with the disease relapse rate decreasing from 34% to 21%:
However, this combination was associated with a significantly worse adverse event profile, with grade 3-4 local toxicity of 28.4% and 55.9% in the mitomycin 10 mg and 30 mg groups, respectively, compared to 11% in the BCG only arm.8
In contrast to these two studies, a 17-year follow-up of the Nordic CIS trial demonstrated that alternating therapy with mitomycin C and BCG did not improve oncologic outcomes compared to BCG monotherapy for patients with CIS. Patients in the alternating therapy arm received six weekly instillations of mitomycin C 40 mg followed by 10 instillations of BCG (Connaught 120 mg) or mitomycin C alternating monthly for 1 year. Patients in the BCG group had a significantly lower 15-year recurrence rate (49% versus 59%; HR 0.74, 95% CI 0.54 to 1.00). There were no significant differences in time to progression and disease-specific or overall survivals.9 It is important to note that there are significant differences between these trials in the eligibility criteria (T1 only versus CIS only versus any intermediate- or high-risk NMIBC) and treatment dosing/schedule, which may explain the observed differences in efficacy/safety outcomes.
MITO-BCG is an ongoing open-label, phase 4 trial that is evaluating the efficacy and safety of sequential mitomycin C and BCG combination treatment in patients with high-risk NMIBC. Patients in Group 1 (n=31) received BCG induction once weekly for six weeks, whereas those in Group 2 (n=41) received additional 40 mg mitomycin instillations the day prior to the BCG instillations. Interim results with a mean follow-up of 71 months were presented at AUA 2023, with no significant differences in recurrence rates (19% versus 24%, p=0.61). Nine patients experienced adverse events with eight Grade 1 and one Grade 4 (orchiectomy) events. No statistically significant differences were recorded in terms of complications between groups.10
To test the hypothesis of whether mitomycin C may act as an ‘immunogenic primer’ by enhancing the expression of cellular antigens that augment subsequent immune responses to BCG, a phase III RCT is randomizing patients with primary treatment NMIBC to either neoadjuvant mitomycin C or standard of care. Patients in the neoadjuvant mitomycin C group will receive two intravesical instillations of mitomycin C (40 mg/40 ml saline) in the 2 weeks before (days: -14 and -7) the scheduled TURBT (day: 0). After the TURBT, both controls and neoadjuvant mitomycin C subjects will undergo adjuvant treatment, as indicated based on the histological evaluation of the tumor and in keeping with the current EAU guidelines. The trial design is as follows:
The primary study endpoint is the complete response rate at 3, 6, 12, and 24 months post-TURBT, with secondary endpoints of rates of grade and stage progression to muscle invasive disease.
Rapamycin + BCG
In preclinical models, rapamycin has been shown to enhance BCG vaccine’s efficacy against tuberculosis and the killing capacity of γδ T cells, which are critical for BCG’s antitumor effects.11 A randomized, double-blind trial evaluated the addition of oral rapamycin (0.5 or 2 mg daily) versus placebo for 1 month in 31 patients with NMIBC receiving 3-weekly BCG instillations. Patients receiving 2 mg rapamycin (n=8) had the most significant increase in BCG-specific γδ T cells from baseline (+78.8% versus +9.6% and -26% in the rapamycin 0.5 mg and placebo groups, respectively). BCG-induced cytokines showed a progressive increase in IL-8 (p=0.02) and TNF-α (p=0.04) over time for patients on rapamycin 2 mg, whereas patients receiving placebo had no significant change in urinary cytokines. Compared with placebo, patients receiving 2 mg rapamycin had increased urinary γδ T cells at the first week of BCG (p=0.02). While efficacy outcomes from this trial are pending, these early results demonstrate that rapamycin may enhance BCG-specific immune responses in patients during the maintenance phase of BCG treatment, suggesting that rapamycin could help provide sustained BCG-mediated immune responses for bladder cancer patients.12
Intravesical Chemotherapy
Gemcitabine + DocetaxelThe intravesical combination of gemcitabine and docetaxel has emerged as one of the most commonly used treatments for patients with BCG unresponsive NMIBC, due to its efficacy, ease of administration, wide availability, and relatively low cost.13 As a result, there has been increased interest in moving up this combination to earlier disease settings, particularly during periods of BCG shortages. The University of Iowa group published their single center experience comparing the outcomes of patients with high-risk, BCG naïve NMIBC receiving gemcitabine + docetaxel versus BCG. Similar to BCG, patients received induction gemcitabine + docetaxel once weekly for six weeks, with subsequent maintenance instillations given once monthly for patients without evidence of disease recurrence. The 6-, 12-, and 24-months high-grade recurrence-free survival estimates in each group were as follows:
- Gemcitabine + docetaxel: 92%, 85%, and 81%, respectively
- BCG: 76%, 71%, and 69%, respectively
Multivariable Cox regression analyses adjusted for differences in patient age, sex, treatment year, and presence of demonstrated that the combination of gemcitabine + docetaxel, compared to BCG, was associated with significantly better high-grade recurrence-free survival rates (HR 0.57; 95% CI 0.33 to 0.97).14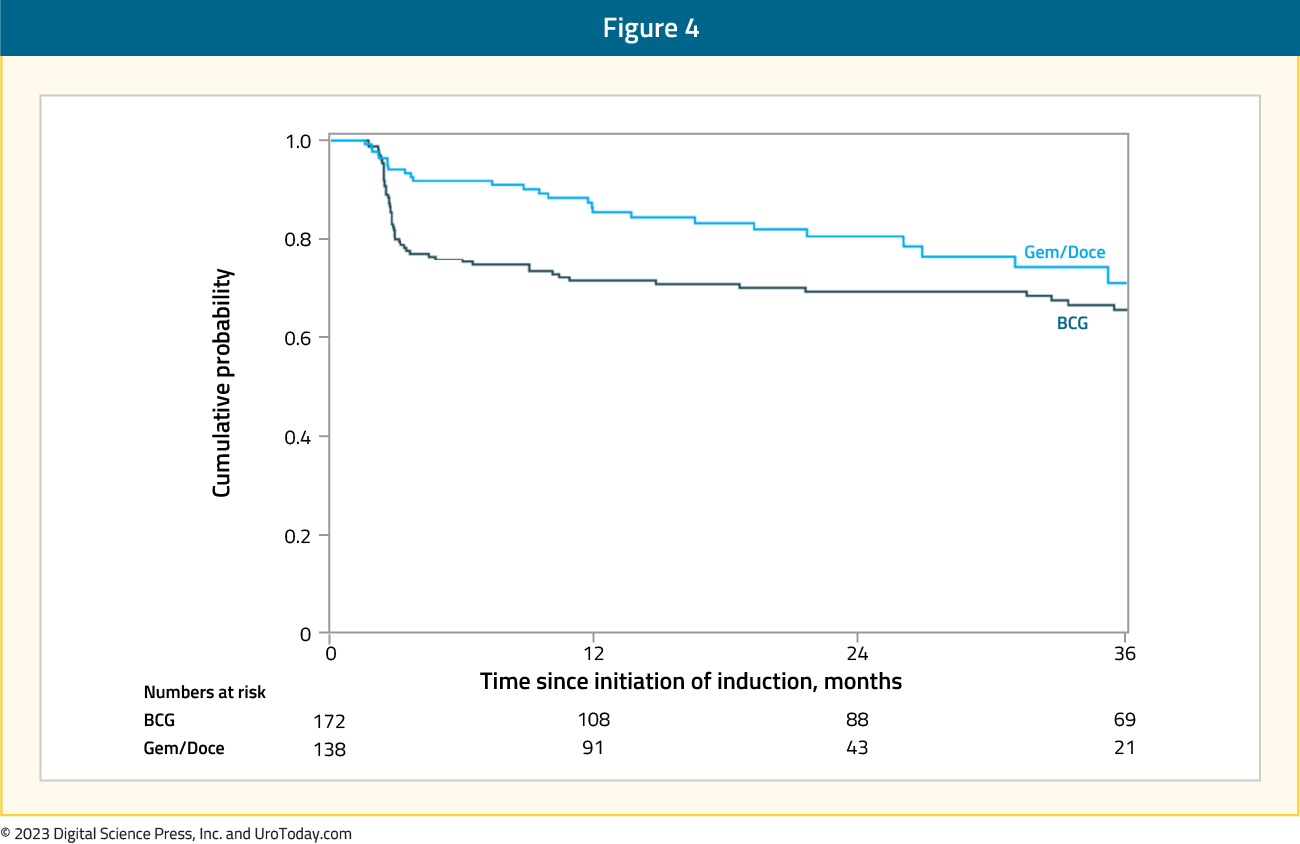
Furthermore, fewer patients receiving induction gemcitabine + docetaxel discontinued therapy during induction. In the absence of level one evidence comparing these two treatment regimens, these results suggest that gemcitabine + docetaxel is a viable alternative to BCG in the BCG naïve setting, during the current BCG shortage.
The ongoing EA8212 BRIDGE study (NCT05538663) is a non-inferiority trial comparing gemcitabine + docetaxel to standard BCG for patients with BCG-naïve high-grade NMIBC. This study will randomize 870 patients 1:1 (stratified by pure CIS, pure papillary, and mixed) to gemcitabine + docetaxel 6 weekly cycles with monthly maintenance versus BCG 6 weekly cycles with SWOG protocol maintenance. The primary outcome for this study is event free survival, defined as the time from randomization to high-grade recurrence in the bladder (CIS, high-grade Ta, high-grade T1 or T2 disease), progression of disease, or death. The study design is as follows:
Gemcitabine or Mitomycin
At AUA 2023, the results of a ‘real world’ analysis comparing intravesical chemotherapy to BCG in patients with newly diagnosed high-grade Ta urothelial carcinoma of the bladder were presented. This study included 166 patients from Memorial Sloan Kettering Cancer Center (MSKCC), who were ‘naturally’ randomized to either BCG (n=111) or chemotherapy (n=55; gemcitabine: 15, mitomycin: 40). During the ongoing shortage, BCG use has been restricted to patients with either HG T1 or CIS only. As such, patients in the high-grade Ta group have been randomly given either BCG or chemotherapy, as per the treating physician’s discretion.
Overall, baseline characteristics, including tumor size and focality, were well-balanced between the two groups. On univariable analysis, there were no significant differences in the primary outcome of recurrence-free survival between the treatment arms (p=0.47). In the BCG group, 88% and 74% were free of any recurrence at 6 and 12 months, respectively, compared to 83% and 76% in the chemotherapy group. The cumulative risk of high-grade recurrences was also similar between both groups, with 78% of patients free of high-grade recurrence at 12 months in the chemotherapy group, compared to 82% in the BCG group. Progression, defined as the presence of ≥T1 disease or undergoing a radical cystectomy, was similar in the two groups (12 months: 89%; p=0.95). These results provide additional support for the safe use of intravesical chemotherapy, instead of BCG availability, for well-selected patients during the ongoing BCG shortage.
Hyperthermic Intravesical Chemotherapy (HIVEC) with Mitomycin CIn 2022, Guerrero-Ramos et al. published results from a phase II trial comparing hyperthermic intravesical chemotherapy (HIVEC) with 40 mg of mitomycin C (once weekly instillations for 6 weeks plus subsequent maintenance) to intravesical BCG for 1 year in patients with mostly BCG-naïve, high-risk NMIBC (excluding CIS). This trial included 25 patients in each arm, with 5 (20%) patients in the BCG arm having received prior intravesical therapy (mitomycin: 4; BCG: 1), compared to none in the HIVEC arm. The 24 months recurrence-free survival rates were non-significantly higher in the HIVEC arm (87% versus 72%, p=0.18), with a median time to recurrence of 21.5 and 16.1 months with HIVEC and BCG, respectively. Notably, patients in the HIVEC arm had significantly improved progression-free survival rates at 96%, compared to 72% (p=0.043) in the BCG arm:
Based on these results, the authors concluded that HIVEC provides comparable safety and efficacy to BCG and is a reasonable alternative during BCG shortages.15
Conclusions
The ongoing BCG shortage has accelerated efforts to find novel intravesical alternatives for patients with newly diagnosed, BCG naïve NMIBC. Based on the currently available data, it appears that intravesical gemcitabine + docetaxel is a safe alternative to BCG with comparable efficacy for the prevention of high-grade recurrences following a TURBT. Conversely, the addition of intravesical mitomycin to BCG may improve oncologic efficacy, albeit with inconsistent results and a potentially worse toxicity profile. Given these limitations, emerging studies in this space are increasingly utilizing novel immune checkpoint inhibitor combinations to enhance the efficacy of BCG for the prevention of subsequent disease recurrence and progression.Published: August 2023


