Library Resources
Library Resources
- Written by: Rashid K. Sayyid, MD MSc, University of Southern California, Los Angeles, CA & Zachary Klaassen, MD, MSc, Wellstar MCG Health, Georgia Cancer Center, Augusta, GA
- References:
- FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer. Accessed on December 12, 2024.
- FDA approves second PSMA-targeted PET imaging drug for men with prostate cancer. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-second-psma-targeted-pet-imaging-drug-men-prostate-cancer#:~:text=FDA%20approved%20the%20first%20PSMA,at%20two%20sites%20in%20California. Accessed on December 12, 2024.
- U.S. FDA Approves Blue Earth Diagnostics’ POSLUMA® (Flotufolastat F 18) Injection, First Radiohybrid PSMA-targeted PET Imaging Agent for Prostate Cancer. https://www.businesswire.com/news/home/20230530005180/en/U.S.-FDA-Approves-Blue-Earth-Diagnostics%E2%80%99-POSLUMA%C2%AE-Flotufolastat-F-18-Injection-First-Radiohybrid-PSMA-targeted-PET-Imaging-Agent-for-Prostate-Cancer. Access on December 13, 2024.
- Afshar-Oromieh A, da Cunha ML, Wagner J, et al. Performance of [68Ga]Ga-PSMA-11 PET/CT in patients with recurrent prostate cancer after prostatectomy-a multi-centre evaluation of 2533 patients. Eur J Nucl Med Mol Imaging. 2021; 48(9):2925-34.
- Vlachostergios PJ, Niaz MJ, Sun M, et al. Prostate-Specific Membrane Antigen Uptake and Survival in Metastatic Castration-Resistant Prostate Cancer. Front Oncol. 2021; 11:630589.
- Huynh TT, van Dam EM, Sreekumar S, et al. Copper-67-Labeled Bombesin Peptide for Targeted Radionuclide Therapy of Prostate Cancer. Pharmaceuticals (Basel). 2022; 15(6):728.
- Tolvanen T, Kalliokoski K, Malaspina S, et al. Safety, Biodistribution, and Radiation Dosimetry of 18F-rhPSMA-7.3 in Healthy Adult Volunteers. J Nucl Med. 2021; 62(5):679-84.
- Wurzer A, Di Caro D, Schmidt A, et al. Radiohybrid ligands: a novel tracer concept exemplified by 18F- or 68Ga-labeled rhPSMA-inhibitors. J Nucl Med. 2020; 61(5):735-42.
- Wurzer A, Parzinger M, Konrad M, et al. Preclinical comparison of four [18F, natGa]rhPSMA-7 isomers: influence of the stereo configuration on pharmacokinetics. EJNMMI Res. 2020; 10(1):149.
- Wurzer A, Kunert J, Fischer s, et al. Synthesis and preclinical evaluation of 177Lu-labeled radiohybrid PSMA ligands (rhPSMAs) for endoradiotherapy of prostate cancer. J Nucl Med. 2022; 63(10):1489-95.
- Jani AB, Ravizzini GC, Gartrell BA, et al. Diagnostic Performance and Safety of 18F-rhPSMA-7.3 Positron Emission Tomography in Men With Suspected Prostate Cancer Recurrence: Results From a Phase 3, Prospective, Multicenter Study (SPOTLIGHT). J Urol. 2023; 210(2):299-311.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase 3, Multicenter Study. Clin Cancer Res. 2021;27(13):3674-82.
- Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer. JAMA Oncol. 2019; 5(6):856-63.
- Fleming MT, Hermsen R, Purysko AS, et al. True-Positive 18F-Flotufolastat Lesions in Patients with Prostate Cancer Recurrence with Baseline-Negative Conventional Imaging: Results from the Prospective, Phase 3, Multicenter SPOTLIGHT Study. J Nucl Med. 2024; 65(7):1080-6.
- Nordquist L, Lengyelova E, Saltzstein D, et al. COBRA: Assessment of safety and efficacy of 64Cu-SAR-bisPSMA in patients with biochemical recurrence of prostate cancer following definitive therapy. J Clin Oncol. 2024; 42:Number 16_suppl.
- Baratto L, Jadvar H, Iagaru A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Mol Imaging Biol. 2018; 20(4):501-9.
- Beer M, Montani M, Gerhardt J, et al. Profiling gastrin-releasing peptide receptor in prostate tissues: clinical implications and molecular correlates. Prostate. 2012; 72(3):318-25.
- Faviana P, Boldrini L, Erba PA, et al. Gastrin-Releasing Peptide Receptor in Low Grade Prostate Cancer: Can It Be a Better Predictor Than Prostate-Specific Membrane Antigen? Front Oncol. 2021; 11:650249.
- Mapelli P, Ghezzo S, Gajate AMS, et al. 68Ga-PSMA and 68Ga-DOTA-RM2 PET/MRI in Recurrent Prostate Cancer: Diagnostic Performance and Association with Clinical and Histopathological Data. Cancers (Basel). 2022; 14(2):334.
- Kurth J, Krause B, Joksch M, et al. Intraindividual comparison of [68Ga]Ga-RM2 and [68Ga]Ga-PSMA PET/CT in patients with mCRPC in a theranostic setting. J Nucl Med. 2023; 64(Supplement 1):1089.
- Nordquist LT, Lengyelova E, Iagaru A, et al. SABRE: Assessment of safety and efficacy of 64Cu-SAR-BBN in patients with PSMA-negative biochemical recurrent prostate cancer. J Clin Oncol. 2024; 42:Number 4_suppl.
New PET Imaging Tracers for the Primary Staging of Unfavorable Intermediate and High-Risk Prostate Cancer
Introduction
In December 2020, Gallium 68 PSMA-11 (68Ga PSMA-11) was approved by the U.S. Food and Drug Administration (FDA) for the initial staging of prostate cancer patients at high risk of metastases,1 and this was shortly followed by the FDA approval of 18F-DCFPyL (PYLARIFY®) in May 2021.2 These positron emission tomography (PET) imaging modalities have since been endorsed by numerous international guidelines and have been widely adopted in clinical practice for the primary staging of unfavorable intermediate- and high-risk prostate cancer patients.- Written by: Rashid K. Sayyid, MD, MSc, University of Southern California Los Angeles, CA and Zachary Klaassen, MD, MSc, Wellstar MCG Health Goergia Cancer Center Augusta, GA
- References:
- FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. Accessed on December 12, 2024.
- FDA approves second PSMA-targeted PET imaging drug for men with prostate cancer. Accessed on December 12, 2024.
- Hofman MS, Lawrentschuk N, Francis, RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet. 2020; 395(10231):1208–16.
- Tolvanen T, Kalliokoski K, Malaspina S, et al. Safety, Biodistribution, and Radiation Dosimetry of 18F-rhPSMA-7.3 in Healthy Adult Volunteers. J Nucl Med. 2021; 62(5):679-84.
- Wurzer A, Di Caro D, Schmidt A, et al. Radiohybrid ligands: a novel tracer concept exemplified by 18F- or 68Ga-labeled rhPSMA-inhibitors. J Nucl Med. 2020; 61(5):735-42.
- Wurzer A, Parzinger M, Konrad M, et al. Preclinical comparison of four [18F, natGa]rhPSMA-7 isomers: influence of the stereo configuration on pharmacokinetics. EJNMMI Res. 2020; 10(1):149.
- Wurzer A, Kunert J, Fischer s, et al. Synthesis and preclinical evaluation of 177Lu-labeled radiohybrid PSMA ligands (rhPSMAs) for endoradiotherapy of prostate cancer. J Nucl Med. 2022; 63(10):1489-95.
- U.S. FDA Approves Blue Earth Diagnostics’ POSLUMA® (Flotufolastat F 18) Injection, First Radiohybrid PSMA-targeted PET Imaging Agent for Prostate Cancer. Accessed on December 12, 2024.
- Surasi DS, Eiber M, Maurer T, et al. Diagnostic Performance and Safety of Positron Emission Tomography with 18F-rhPSMA-7.3 in Patients with Newly Diagnosed Unfavourable Intermediate- to Very-high-risk Prostate Cancer: Results from a Phase 3, Prospective, Multicentre Study (LIGHTHOUSE). Eur Urol. 2023; 84(4): 361-70.
- Pienta KJ, Gorin MA, Rowe SP, et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18 F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol 2021; 206(1):52-61.
- Hope TA, Eiber M, Armstrong WR, et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021; 7(11):1635-42.
- Kuo P, Ravizzini G, Ulaner G, Yoo D, Zukotynski K. Inter- and intra-reader reproducibility of 18F-rhPSMA-7.3 PET interpretation in patients with newly diagnosed prostate cancer: Results from a phase 3, prospective, multicenter study (LIGHTHOUSE). J Nucl Med. 2023; 64(Supplement 1):589.
- Lengyelova E, Wong V, Lenzo N, Parker M, Emmett L. 64Cu-SAR-bisPSMA (PROPELLER) positron emission tomography (PET) imaging in patients with confirmed prostate cancer. J Clin Oncol. 2023; 41:Number 16_suppl.
- Gorin MA, Lengyelova E, Nordquist L, et al. CLARIFY: Positron emission tomography using 64Cu-SAR-bisPSMA in patients with high-risk prostate cancer prior to radical prostatectomy (a phase 3 diagnostic study). J Clin Oncol. 2024; 42:Number 16_suppl.
Bipolar Androgen Therapy: Rationale, Candidate Patients, and Latest Evidence for mCRPC Patients
Androgen receptor inhibition remains the mainstay treatment of advanced prostate cancer. However, therapeutic resistance to androgen receptor inhibition is almost universal. Prostate cancer cells can develop resistance to androgen ablation through an adaptive marked upregulation of androgen receptors over time in response to low-androgen conditions. This upregulation can make these cells vulnerable to supraphysiologic testosterone exposure. Bipolar androgen therapy (BAT) has been proposed as an approach to overcome androgen receptor therapeutic resistance. Rapid cycling between polar extremes of supraphysiologic and near-castrate serum testosterone in asymptomatic men with metastatic castrate-resistant prostate cancer (mCRPC) has been proven safe and effective. There are five unique features to BAT:
- Written by: Rashid K. Sayyid, MD, MSc, University of Southern California Los Angeles, CA & Zachary Klaassen, MD, MSc, Wellstar MCG Health Augusta, GA
- References:
- Linja MJ, Savinainen KJ, Saramaki OR, et al. Amplification and overexpression of AR gene in hormone-refractory PCa. Cancer Res. 2001;61:3550-3555.
- Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant PCa. Cancer Res. 2009;69:2912-2918.
- Brown RS, Edwards J, Dogan A, et al. Amplification of the AR gene in bone metastases from hormone-refractory PCa. J Pathol. 2002;198:237-244.
- Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in PCa. N Engl J Med. 2014;371:1028-1038.
- Isaacs JT, D’Antonio JM, Chen S, et al. Adaptive auto-regulation of AR provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human PCa. Prostate. 2012;72:1491-1505.
- Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33-39.
- Isaacs JT, D’Antonio JM, Chen S, et al. Adaptive auto-regulation of AR provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human PCa. Prostate. 2012;72:1491-1505.
- Chatterjee P, Schweizer MT, Lucas JM, et al. Supraphysiological androgens suppress PCa growth through AR-mediated DNA damage. J Clin Invest. 2019;130:127613.
- Chuu CP, Hiipakka RA, Fukuchi J, Kokontis JM, Liao S. Androgen causes growth suppression and reversion of androgen-independent PCa xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005;65:2082-2084.
- Denmeade SR, Sena LA, Wang H, et al. Bipolar Androgen Therapy Followed by Androgen Receptor Inhibition as Sequential Therapy for Prostate Cancer. Oncologist. 2023;28(6):465-73.
- Denmeade SR, Wang H, Agarwal N, et al. TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer. J Clin Oncol. 2021;39(12):1371-82.
- Markowski MC, Wang H, Sullivan R, et al. A Multicohort Open-label Phase II Trial of Bipolar Androgen Therapy in Men with Metastatic Castration-resistant Prostate Cancer (RESTORE): A Comparison of Post-abiraterone Versus Post-enzalutamide Cohorts. Eur Urol. 2021;692-699.
- Mendonca J, Kumar R, Owoyemi O, et al. Supraphysiological testosterone induces ferroptosis and activates NF-kB mediated immune pathways in PCa through nucleophagy. Cancer Res. 2021;81(23):5948-5962.
- Markowski MC, Shenderov E, Eisenberger MA, et al. Extreme responses to immune checkpoint blockade following bipolar androgen therapy and enzalutamide in patients with metastatic castration resistant PCa. Prostate. 2020;80:407-411.
- Markowski MC, Talpin ME, Aggarwal R, et al. Bipolar androgen therapy plus nivolumab for patients with metastatic castration-resistant prostate cancer: the COMBAT phase II trial. Nat Commun. 2024;15(1):14.
- Schweizer MT, Gulati R, Yezefski T, et al. Bipolar androgen therapy plus olaparib in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2023;26(1):194-200.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed on August 11, 2024.
IBCN 2024: Highlights
Promoting BCAN’s 2025 Walks to End Bladder Cancer: Join the Fight Against Bladder Cancer
Highlights from ESMO’24 Presidential Symposium: Bladder Cancer in the Forefront
Systemic Treatment in Bladder Cancer State of Art in 2024 and Challenges in Morocco
Bladder cancer treatment has evolved from traditional surgery and chemotherapy to include immunotherapy, targeted therapies, and antibody drug conjugates. These therapeutic innovations, along with advances in surgical techniques and multimodal approaches, continue to reshape clinical practice and improve outcomes for bladder cancer patients. However, the high cost of these treatments poses a significant challenge in low-income countries such as Morocco.
IBCG Retreat 2024
A New Horizon in BCG Unresponsive Non-Muscle Invasive Bladder Cancer: Anktiva (N-803) + BCG
Introduction
Intravesical Bacillus Calmette-Guerin (BCG) currently remains the standard of care, guideline recommended treatment of choice in the adjuvant setting for intermediate- and high-risk non-muscle invasive bladder cancer (NMIBC) due to its ability to reduce the risk of disease recurrence and disease progression.1-3 However, despite adequate BCG, up to 50% of patients develop a BCG-refractory, relapsing, or failure disease state.4 Currently, radical cystectomy remains the gold standard approach in this setting.1 However, many patients are either unfit or refuse cystectomy.
Given the shortage of options in this disease space, along with the modest efficacy of Valstar (21% complete response with a median response duration of one year),5 in 2018 the FDA issued a guidance document to promote the development of drugs and biologics for BCG-unresponsive NMIBC:6
- Persistent or recurrent CIS within 12 months of adequate BCG therapy
- Recurrent high-grade Ta/T1 disease within six months of completion of adequate BCG therapy
- High-grade T1 disease detected on the first evaluation following an induction BCG course
The focus of this Center of Excellence article is to highlight the currently available evidence and ongoing studies for Nogapendekin alfa-inbakicept (NAI; Anktiva, ie. N-803) in NMIBC in the BCG-unresponsive disease space, as well as other ongoing trials in progress for other indications.
QUILT-3.032: Initial Results
Impaired T-cell and cytotoxic cellular response play a key role in the development of BCG failure for patients with NMIBC. N-803 is an interleukin-15 (IL-15) superagonist that acts as an activation and proliferation factor for natural killer and effector/memory T-cells:

Given that BCG has been shown to establish ‘trained immunity’ as the molecular basis for its immunotherapeutic effect in bladder cancer, it has been hypothesized that N-803 could further enhance the immune response by mediating a second, unrelated stimulus.
QUILT-3.032 is an ongoing, open label, multicenter, single arm trial that is evaluating intravesical N-803 + BCG or N-803 alone in patients with BCG-unresponsive, high-grade NMIBC7. This study includes three patient cohorts8:
- Cohort A (CIS +/- papillary disease): Intravesical N-803 (400 μg/instillation) + BCG (50 mg/installation) given once weekly for 6 consecutive weeks (induction)
- Cohort B (High grade Ta/T1 papillary disease): N-803 + BCG
- Cohort C (CIS +/- papillary): N-803 alone

In this trial, surveillance included a cystoscopy + urinary cytology every 3 months for the first 24 months, followed by every six months thereafter until 60 months. Importantly, biopsy was mandated at week 12 in all patients, irrespective of cystoscopy or cytology findings. Re-induction was offered to patients without a complete response, conditional on the absence of T1 or worse disease in the specimen. These patients would then undergo a mandatory 6-month biopsy. The primary endpoints in Cohorts A and C were 3- and 6-month complete response, defined by one of the following three scenarios:
- Negative cystoscopy and cytology (including atypical cytology)
- Biopsy-proven benign or low-grade Ta disease and negative cytology
- Negative cystoscopy with malignant urinary cytology, if cancer found in the upper tract or prostatic urethra and random bladder biopsies were negative
In Cohort A (n = 82), a complete response was observed in 71% of patients (13/58 responders required a re-induction). Response rates were as follows:
- 3 months: 55%
- 6 months: 56%
- 12 months: 45%
The median duration of response in complete responders was 26.6 months. The 24-month progression-free, overall, and disease-specific survival rates were 85%, 94%, and 100% respectively.

In 72 evaluable patients in Cohort B, the median disease-free survival was 19.3 months (95% CI 7.4 – NR), with disease-free survival rates as follows:
- 12 months: 55.4% (95% CI 42.0 – 66.8)
- 18 months: 51.1% (95% CI 37.6 – 63.1)
- 24 months: 48.3% (95% CI 34.5 – 60.7)

The 24-months progression-free, overall, and disease-specific survival rates were 88.8%, 91.7%, and 97.7%, respectively.
In the 10 evaluable patients in Cohort C, a complete response at 3 months was observed in only 2 (20%) patients with N-803 alone. Of the 8 initial non-responders, 6 underwent re-induction with only 1 of these 6 patients demonstrating evidence of a complete response at 6 months. On the basis of protocol-defined stopping rules, the independent data monitoring committee recommended that cohort C be discontinued for futility.
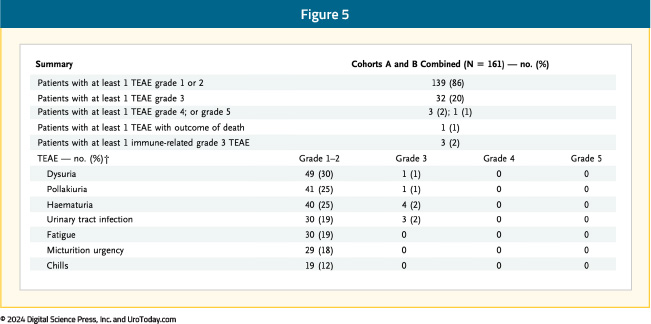
The most common treatment-related adverse events with the combination of N-803 + BCG were lower genitourinary in nature (dysuria, pollakiuria, hematuria). The incidence of grade 3 or worse adverse events was 23%, most frequently hematuria and urinary tract infections (2% each). One patient experienced a grade 5 treatment-related adverse event (cardiac arrest with subsequent death) and 3 had evidence of immune-related adverse events:7
QUILT-3.032: After the Initial Results
FDA Approval
On April 22, 2024, the Food and Drug Administration (FDA) approved N-803 + BCG for patients with BCG-unresponsive NMIBC with CIS +/- papillary tumors based on results from the QUILT-3.032 trial.
Quality of Life Assessment
In Cohort A, the mean baseline ECOG score was 0.183, with 82% of patients having a score of 0. Quality of life was measured by the EORTC QOL Questionnaire Core 30 (QLQ-C30) and QOL NIMBC-Specific 24 Questionnaire (QLQ-NMIBC24). During the study, hospitalizations for any reason remained low (0% - 6% per assessment). Moreover, participant quality of life from completion rate was high, with 90% or greater at most time points. There was a modest decrease in mean physical function and global health from baseline at all assessed on-study time points that became less by week 104: 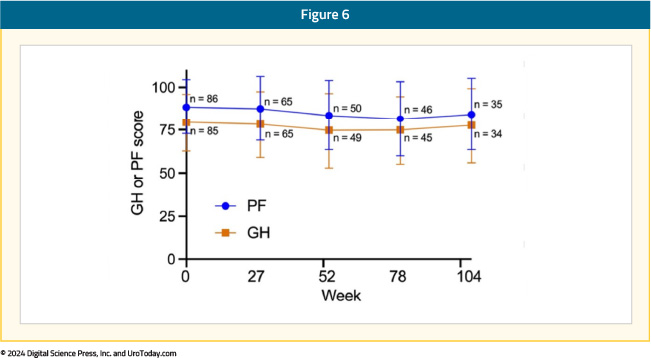
When responders (those with a complete response) were compared with non-responders, they showed less of a decrease in physical function and global health scores with time, although both parameters were higher at baseline for responders:

At month 12, >3 prior TURBTs were significantly associated (p = 0.0729) with lower global health scores as compared to <= prior TURBTs. At month 6, achievement of complete response was associated (p = 0.0659) with higher physical function scores as compared to no complete response. No other baseline variable had a significant level of association:

QUILT-3.032 Updated Results
At AUA 2024, Dr. Patrick Soon-Shiong presented updated results of the QUILT-3.032 trial. At the time of the data cutoff of November 30, 2023, patients with an ongoing complete response at month 25 and later, maintenance instillations were administered once a week for 3 weeks at months 25, 31, and 37 for a maximum of 9 additional instillations. In this highly pre-treated group, the complete response rate was 65%. Among patients who had received ≥12 prior BCG doses (n = 74), the complete response rate was 69%. Conversely, among those who received <12 doses of prior BCG, the complete response rate was 70%. A total of 53 patients were BCG-unresponsive/BCG-relapsed, and the complete response rate in this group was 72%. Thus, both BCG-unresponsive/BCG-refractory and BCG-unresponsive/BCG-relapsed had complete response rates comparable to the efficacy population. The median duration of response was 26.6 months (95% CI: 0.9 – not reached). By the Kaplan-Meier method, 61.3% of subjects had a duration of complete response lasting >12 months and 53.2% had a duration of >24 months.
Looking Ahead: QUILT-2.005 in BCG-Naïve Patents
Also at AUA 2024, Dr. Sandeep Reddy discussed the QUILT-2.005 trial comparing intravesical BCG in combination with N-803 to BCG alone in patients with BCG-naïve NMIBC. In phase 1 of this trial, there was durable complete remission among 9 patients with CIS and papillary NMIBC treated with BCG + N-803. As requested by the Agency, there was an unplanned interim analysis of the efficacy results in CIS patients for the phase 2 QUILT-2.005 trial, which showed an improvement in complete response rate over time and contribution of effect of N-803 inducing memory T-cells
The trial design for the phase IIb QUILT-2.005 is for participants to be randomized 1:1 to one of two independent treatment arms based on disease and ECOG status. Participants receive either intravesical 400 ug N-803/instillation + 50 mg BCG or BCG alone weekly for six consecutive weeks, followed by maintenance treatment for 3 consecutive weeks at 3, 6, 12, and 18 months. At 3 months, re-induction with another 6 weeks of therapy for eligible patients is an option. Cohort A of QUILT-2.005 will have CIS +/- Ta or T1 disease patients, and Cohort B will have high grade papillary Ta or 1 disease patients. Participants must have a diagnostic biopsy within 3 months of treatment start and a cystoscopy demonstrating no resectable disease within 6 weeks. Participants must be BCG-naïve (or last BCG > 3 years prior) and have not received more than a single post-operative dose of mitomycin C or gemcitabine following the most recent screening TURBT/biopsy.
The primary outcomes of QUILT-2.005 are complete response rate and disease free survival with the time frames of 12 and 24 months, respectively. The complete response rate for Cohort A and disease free survival for Cohort B will be compared between treatment arms using cystoscopy, confirmatory biopsy and urine cytology. Safety will be assessed by number/severity of treatment related adverse events over 48 months. As of April 2024, cohort A had enrolled 121 of 366 planned participants, and cohort B had enrolled 74 of 230 planned participants.
N-803 + BCG Logistics and Insurance Coverage
In May 2024, it was announced that ImmunityBio had signed a global agreement with Serum Institute of India (the world’s largest manufacturer of vaccines) to supply ImmunityBio with BCG. The agreement covers manufacturing of standard BCG (approved outside of the US), as well as next generation recombinant BCG (undergoing testing), for the intended use in combination with N-803. Importantly, the aim of this deal is to increase the available supply of BCG and address shortages for the combination with N-803. Furthermore, there are plans to conduct clinical trials to study recombination BCG and standard BCG manufactured by Serum Institute in combination with N-803 for NMIBC.
As of August 2024, N-803 is approved for coverage by more than a dozen insurance plans (corresponding to ~100 million individuals), only months after the FDA approval in the US. Additionally, ImmunityBio has filed for EMA regulatory approval in the European Union, as well as approval to enroll patients in India for the QUILT-2.005 trial. Plans are also underway to apply for approval in South Africa to enroll patients in QUILT-2.005.
Conclusions
Results from the QUILT-3.032 trial in BCG-unresponsive NIMBC have led to the FDA approval of N-803 + BCG in 2024. Importantly, this combination therapy is now approved by several insurance plans and has been recognized as a therapeutic option in the NCCN guidelines. In light of the global BCG shortages, ImmunityBio has successfully partnered with Serum Institute of India to increase BCG availability. Results of the ongoing QUILT-2.005 trial in BCG naïve NMIBC are eagerly anticipated, with continued site expansion in the US, as well as India and potentially South Africa.
Published November 2024
- Written by: Zachary Klaassen, MD, MSc, Wellstar MCG Health, Georgia Cancer Center, Augusta, Georgia, USA
- References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer. https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Schmidt S, Kunath F, Coles B, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Review. 2020;1(1):CD011935.
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 Update. Eur Urol. 2019;76(5):639–57.
- Steinberg R, Bahnson R, Brosman S, et al. Efficacy and Safety of Valrubicin for the Treatment of Bacillus Calmette-Guerin Refractory Carcinoma in Situ of the Bladder. J Urol. 200;163(3):761-7.
- Bacillus Calmette-Guérin-unresponsive nonmuscle invasive bladder cancer: developing drugs and biologics for treatment guidance for industry. 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bacillus-calmette-guerin-unresponsive-nonmuscle-invasive-bladder-cancer-developing-drugs-and.
- Chamie K, Chang SS, Kramolowsky E, et al. IL-15 Superagonist NAI in BCG-Unresponsive Non–Muscle-Invasive Bladder Cancer. N Engl J Med Evid. 2023;2(1).
- Chamie K, Chang SS, Rosser CJ, et al. N-803 plus BCG Treatment for BCG-Naïve or – Unresponsive Non-Muscle Invasive Bladder Cancer: A Plain Language Review. Future Oncol. 2024;20(31):2307-2317.
Treatment Intensification for High-Risk Biochemically Recurrent M0 HSPC: EMBARK in the PSMA PET Era and Ongoing Trials
Introduction
In November 2023, the United States Food and Drug Administration (FDA) approved enzalutamide, with or without concurrent leuprolide therapy, for conventional imaging-defined non-metastatic hormone-sensitive prostate cancer (M0 HSPC) patients with biochemical recurrence at high risk for metastasis.1 This drug approval followed the publication of the EMBARK trial, a randomized phase III trial of biochemically recurrent patients who had high-risk disease, defined by a PSA doubling time (PSADT) ≤9 months and a PSA level of ≥2 ng/mL above nadir following radiation therapy or ≥1 ng/mL after radical prostatectomy, with or without postoperative radiation therapy. Patients in this trial were randomized 1:1:1 to combination enzalutamide + leuprolide, leuprolide monotherapy, or enzalutamide monotherapy. This trial met its primary endpoint of improved 5-year metastasis-free survival with the combination of enzalutamide + leuprolide (87.3% versus 71.4% for leuprolide alone; HR 0.42, p < 0.001). Similarly, enzalutamide monotherapy was associated with superior 5-year metastasis-free survival rates, compared to leuprolide monotherapy (80% versus 71%; HR 0.63, p = 0.005).2
However, the ‘elephant in the room’ is that patients in the EMBARK trial, who were enrolled between January 2015 and August 2018, were staged using conventional imaging, as opposed to prostate-specific membrane antigen (PSMA) positron emission tomography (PET), which has emerged as a standard of care imaging modality for biochemically recurrent patients, owing to its improved sensitivity for detecting metastatic disease in this setting.3,4 As such, many experts in the field have suggested that there is a ‘discord’ between the study design of EMBARK and contemporary practice.
In this Center of Excellence article, we will discuss the results of the EMBARK trial within the context of contemporary PSMA PET imaging and discuss ongoing trials of biochemically recurrent M0 HSPC patients.
PSMA PET Findings in an ‘EMBARK-like’ Cohort
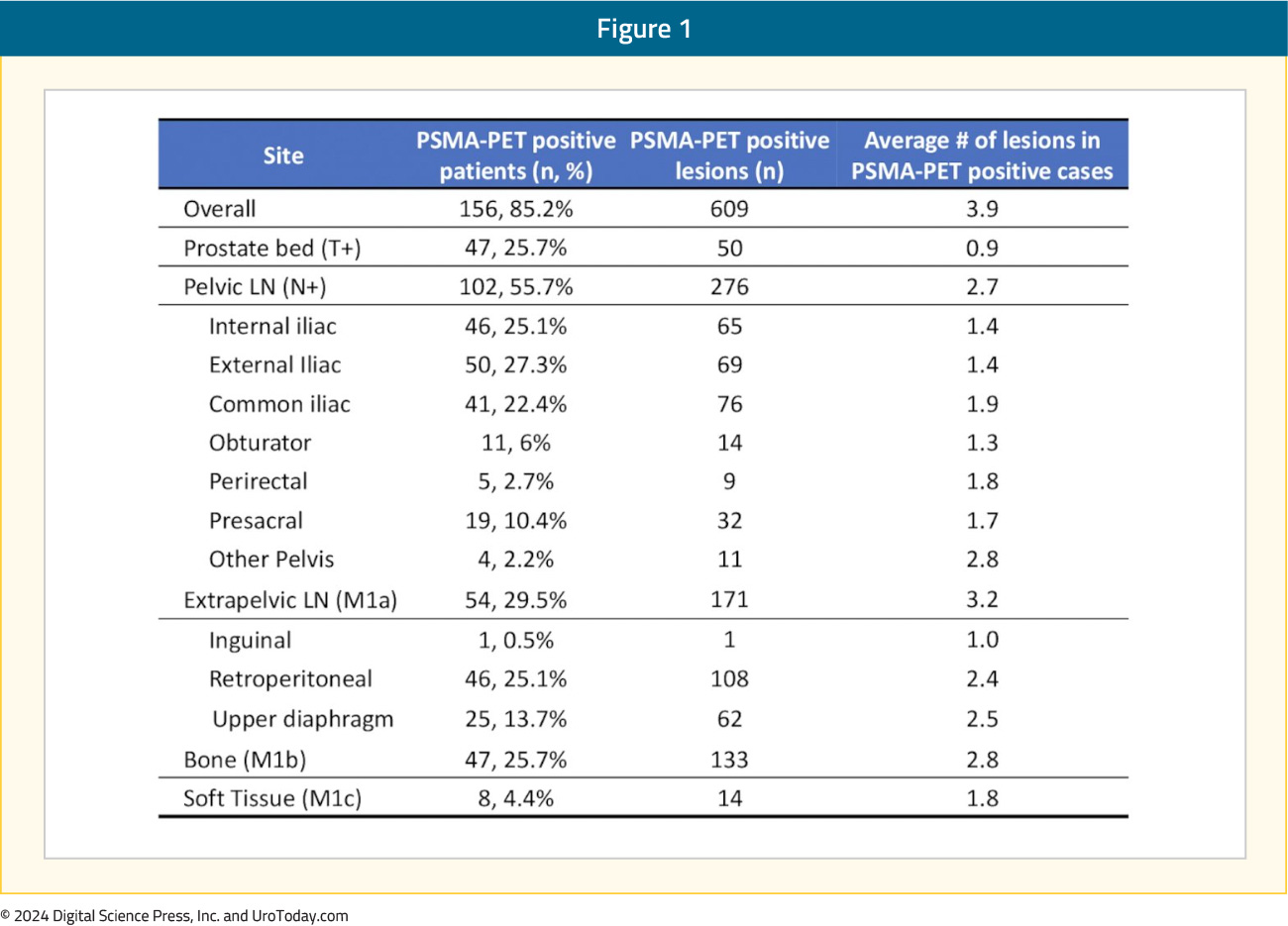
Had trial participants in EMBARK undergone a PSMA PET at study inclusion, what would a PSMA PET scan have shown? At ASCO 2023, Armstrong et al. presented the results of a post-hoc, retrospective analysis of four prospective studies of PSMA PET conducted at UCLA between 2016 and 2021 that included high-risk biochemically recurrent prostate cancer patients who would have met the EMBARK trial eligibility criteria. The study cohort included 183 patients, with a median time from primary therapy to PSMA PET of 39 months. The median serum PSA level at PSMA PET was 2.8 ng/mL, and the median PSADT was 3.6 months.
Overall, 85% of patients were PSMA PET positive, with an average of 3.9 lesions per case. Pelvic nodal disease was present in 56% of patients, and 30% had evidence of extra-pelvic nodal disease. Bone lesions were present in 26% of patients, and 4.4% of patients had visceral metastases:
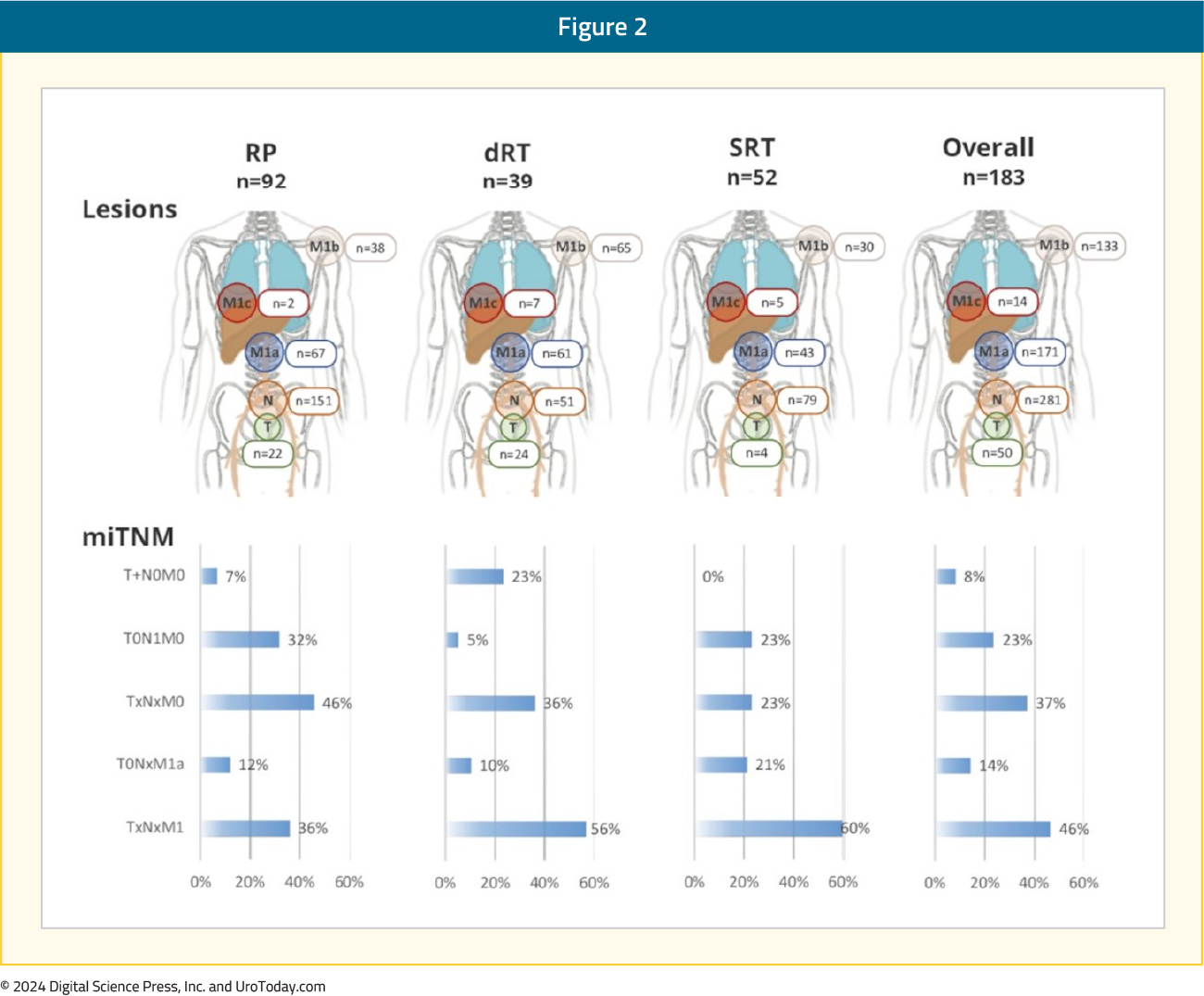
Overall, 23% of patients had evidence of nodal-only disease (i.e., TanyN1M0), 46% had distant metastases (TanyNanyM1), 37% of patients had oligometastatic disease, and 9% had polymetastatic disease (i.e., >5 M1 lesions).5 A visual distribution of the metastatic breakdown is shown below:
Metastasis-Directed Therapy or Systemic Therapy Intensification for ‘EMBARK-like’ Patients
One of the proposed treatment paradigms for the contemporary management of an ‘EMBARK-like’ patient is obtaining a PSMA PET scan and targeting PET-avid lesions with metastasis-directed therapy (MDT), particularly for patients with evidence of oligometastases (i.e., ≤5 lesions), to maximize the systemic therapy-free interval. There are, however, important differences between the EMBARK cohort and the three major phase II trials of MDT in oligorecurrent hormone sensitive prostate cancer: STOMP, ORIOLE, and EXTEND.6,7,8 The major difference is that patients in STOMP and ORIOLE had evidence of metastatic disease on non-PSMA PET imaging (STOMP: choline PET/CT; ORIOLE: conventional imaging [CT, MRI, and/or bone scan]), whereas those in EMBARK had no evidence of metastasis on conventional imaging.
It is important to note, however, that approximately 40% of patients of ‘EMBARK-like’ patients will have oligometastatic disease on a PSMA PET. There is evidence from the ORIOLE trial to support MDT targeting of all PSMA avid lesions. In this trial, pre-treatment 18F-DCFPyL-PET/CT was performed in all patients assigned to the MDT arm (n = 36), and the treating physicians and patients were blinded to the results of these scans. Sixteen patients (44%) had baseline PET-avid lesions that were not included in the MDT treatment fields and had significantly worse 6-month progression rates of 38% (95% CI 18.5–61.5%) compared to those without untreated lesions (5%; 95% CI 0–26.8%; p = 0.03). Furthermore, those with untreated sites of disease had higher rates of new metastases (per conventional imaging) at 6 months (62.5% versus 15.8%, p = 0.006) and worse median distant metastasis-free survival of 6 versus 29 months (HR: 0.19; 95% CI: 0.07–0.54, p < 0.001).6
What about the use of MDT with systemic hormonal therapy? The phase II EXTEND trial demonstrated that the addition of MDT to 6 months of intermittent hormonal therapy significantly improved eugonadal progression-free survival (HR 0.32, p = 0.03). However, similar to the ORIOLE and STOMP, patients had evidence of metastatic disease, mostly identified on conventional imaging (75%). Additionally, approximately 90% of patients had a serum PSA level <2 ng/ml at study entry, whereas the median serum PSA level in EMBARK was 5–5.5 ng/ml. Notably, only 72% of the EXTEND trial patients had undergone definitive local therapy to the prostate, whereas all patients in EMBARK had undergone a radical prostatectomy and/or pelvic radiotherapy, with 50% having undergone both treatment modalities. As such, there are clear differences between the two study cohorts that limit trial comparisons and the generalizability of results and treatment approach used in EXTEND to EMBARK trial participants.
What about the use of MDT with androgen receptor pathway inhibitors? To date, there are no randomized trials comparing the added benefit of combining one modality with the other (ARTO trial of abiraterone +/- stereotactic body radiotherapy [SBRT] was in the castrate-resistant setting) in this disease space.9 The only published trial of combination MDT with androgen receptor pathway inhibitors in oligorecurrent metastatic prostate cancer patients is the phase II SATURN trial, in which patients received six months of leuprolide + abiraterone acetate/prednisone + apalutamide and SBRT to all metastases, with or without radiotherapy directed to the prostate bed and pelvic lymph nodes, after the first month of systemic therapy. Overall, 50% of patients maintained a PSA <0.05 ng/mL six months after testosterone recovery (primary endpoint), and the median eugonodal progression-free survival was 11.4 months.10
One pragmatic approach to treating an ‘EMBARK-like’ patient that has PSMA PET findings of metastatic disease (ie. conventional imaging negative, PSMA PET positive for metastatic disease) is to treat these patients with enzalutamide + leuprolide, rather than enzalutamide monotherapy or leuprolide monotherapy. The rationale for such an approach is that these patients are more “ENZAMET/ARCHES-like” patients, where there is a proven therapeutic benefit of enzalutamide + ADT versus ADT alone in the mHSPC setting.11,12
In summary, the only available level one evidence to inform the treatment of high-risk, biochemically recurrent M0 HSPC patients staged with conventional imaging comes from the EMBARK trial, where both enzalutamide combination therapy with leuprolide and enzalutamide monotherapy have demonstrated metastasis-free survival benefits. Future randomized trials in this space may provide further answers to the question of optimal management of these patients who have PSMA PET positive disease. For the time being, however, similar to the mHSPC treatment paradigm which relies on conventional imaging volume findings to dictate treatment recommendations, high-risk, biochemically recurrent patients with conventional imaging-defined M0 HSPC should be recommended to receive systemic therapy with androgen receptor pathway inhibitors.
Ongoing Trials Utilizing PSMA PET in the High-Risk, Biochemically Recurrent M0 HSPC Space
ARASTEPARASTEP (NCT05794906) is an ongoing randomized, phase III, double-blind, placebo-controlled trial of prostate cancer patients with high-risk biochemical recurrence, defined by a PSADT <12 months with PSA ≥ 0.2 ng/mL after primary radical prostatectomy (+/- adjuvant radiotherapy/salvage radiotherapy) or PSA ≥2 ng/mL above nadir after primary radiotherapy only, ≥1 PSMA PET/CT-positive lesion of prostate cancer without visible lesions on conventional imaging, and serum testosterone >150 ng/dL.
Approximately 750 patients from 184 sites worldwide will be randomized to oral darolutamide 600 mg twice daily or placebo, both with ADT, for 24 months, unless there is earlier evidence of disease progression, unacceptable toxicity, or any other withdrawal criteria are met. Of note, patients will suspend treatment after 24 months if serum PSA levels remain undetectable (i.e., <0.2 ng/mL). Patients whose PSA values remain detectable (i.e., ≥0.2 ng/mL) will continue with the study treatment until PSMA PET/CT progression. The trial design for ARASTEP is as follows:
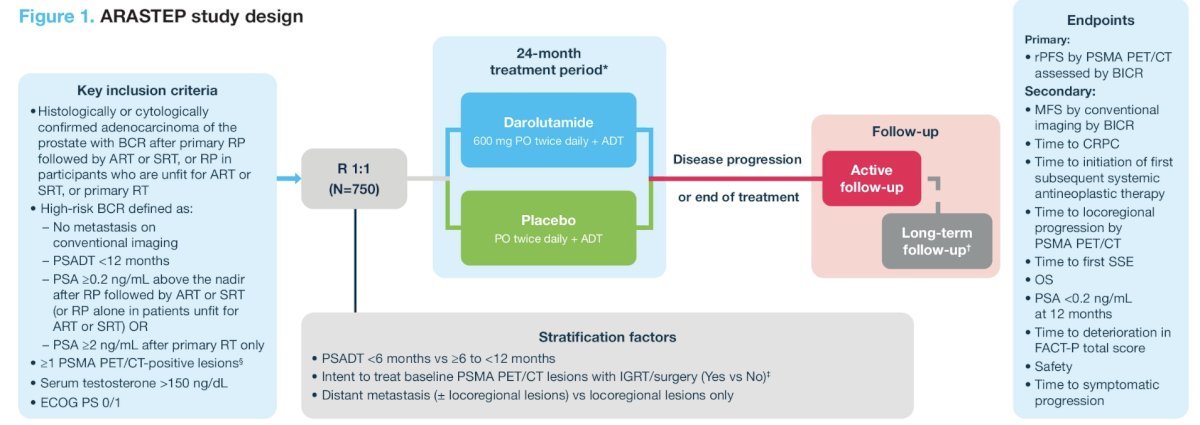
The primary study endpoint is radiographic progression-free survival by PSMA PET/CT, assessed by a blinded independent central review. Key secondary endpoints include:
- Metastasis-free survival by blinded independent central review
- Time to CRPC
- Overall survival
- Quality of life
- Safety
INDICATE (ECOG-ACRIN EA8191; NCT04423211) is a phase III trial that aims to evaluate the following in post-radical prostatectomy biochemically recurrent patients without evidence of metastases on conventional imaging:13
- Whether the addition of apalutamide to standard salvage radiotherapy plus short-term ADT improves survival in patients without extra-pelvic PET-detected lesions
- Whether the addition of MDT to the treatment intensification combination of salvage radiotherapy, short-term ADT, and apalutamide improves survival in patients with PET-detected lesions outside the pelvis
Eligible patients are those with biochemical recurrence post-radical prostatectomy, defined by a serum PSA level >0.5 ng/ml or >0.2 ng/ml, if detected within 12 months of the radical prostatectomy, and no evidence of extra-pelvic metastases on conventional imaging, who are candidates for standard of care salvage therapy (salvage radiotherapy to the prostate bed and pelvic node with concurrent short-term ADT). Of note, if a patient has a detectable PSA (≥0.01 ng/ml) at any time after radical prostatectomy and has a baseline PET that is positive for ≥1 lesion in any location, there is no minimum PSA requirement.
All patients will undergo baseline PET using one of the three FDA-approved tracers, at which point patients will be assigned to either Cohort 1 (PET negative for extra-pelvic metastases) or Cohort 2 (PET positive for extra-pelvic metastases). The study design is as follows:
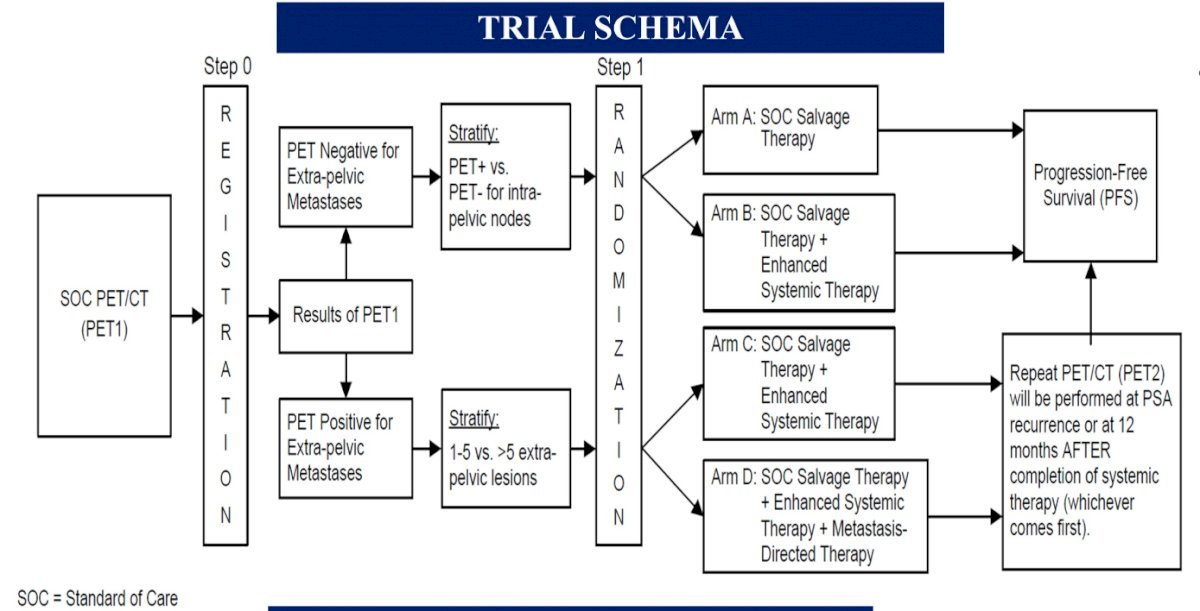
Patients in Cohort 1 (PET negative) will be randomized to either:
- Arm A: Salvage external beam radiotherapy (EBRT) + short-term ADT (leuprolide or goserelin) OR
- Arm B: Salvage EBRT + short-term ADT + apalutamide
Patients in Cohort 2 (PET positive for extra-pelvic metastases) will be randomized to either:
- Arm C: Salvage EBRT + short-term ADT + apalutamide OR
- Arm D: Salvage EBRT + short-term ADT + apalutamide + MDT (SBRT)
The primary endpoint in both cohorts is progression-free survival, defined as time from randomization to radiographic progression on conventional imaging, symptomatic disease progression, or death, whichever occurs first. Secondary endpoints include overall and event-free survival, toxicity, PET progression, and quality of life. The study accrual start date was October 2022, with an estimated primary completion date of December 2027.
Conclusions
Based on the current body of evidence, high-risk, biochemically recurrent patients without evidence of metastases on conventional imaging should be strongly considered for systemic therapy with enzalutamide combination therapy with leuprolide or monotherapy. Despite PSMA PET detecting extra-pelvic metastases in almost 50% of such patients, there is no high-level evidence, to date, to support the routine utilization of PSMA PET findings to direct treatment with SBRT. Ongoing trials will help address important remaining questions in this disease space, namely the value/utility of PSMA PET in patients with negative conventional imaging findings and the added benefit of PSMA PET-directed SBRT to systemic therapy intensification with androgen receptor pathway inhibitors in patients with PSMA PET/conventional imaging discordant findings.
Published November 2024
- Written by: Rashid K. Sayyid, MD MSc University of Southern California Los Angeles, CA & Zachary Klaassen, MD MSc Wellstar MCG Health Augusta, GA
- References:
- FDA approves enzalutamide for non-metastatic castration-sensitive prostate cancer with biochemical recurrence. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enzalutamide-non-metastatic-castration-sensitive-prostate-cancer-biochemical-recurrence. Accessed on August 31, 2024.
- Freedland SJ, de Almeida Luiz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med. 2023; 389(16):1453–65.
- Calais J, Ceci F, Eiber M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20(9):1286-94.
- Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer. JAMA Oncol. 2019; 5(6):856-63.
- Armstrong WR, Clark KJ, Smith CP, et al. PSMA PET findings in an “EMBARK-like” cohort of patients with high-risk non-metastatic hormone-sensitive prostate cancer: A single center post-hoc retrospective analysis. J Clin Oncol. 2023; 41:Number 16_suppl.
- Philips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-9.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:6_suppl.
- Tang C, Sherry AD, Haymaker C, et al. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer: The EXTEND Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023;9(6): 825-34.
- Francolini G, Allegra AG, Detti B, et al. Stereotactic Body Radiation Therapy and Abiraterone Acetate for Patients Affected by Oligometastatic Castrate-Resistant Prostate Cancer: A Randomized Phase II Trial (ARTO). J Clin Oncol. 2023; 41(36):5561-8.
- Nikitas J, Rettig M, Shen J, et al. Systemic and Tumor-directed Therapy for Oligorecurrent Metastatic Prostate Cancer (SATURN): Primary Endpoint Results from a Phase 2 Clinical Trial. Eur Urol. 2024; 85(6):517-20.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019; 381(2):121-131.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019; 37(32):2974-2986.
- Vapiwala N, Chen Y, Cho SY, et al. Phase III study of local or systemic therapy intensification directed by PET in prostate cancer patients with post-prostatectomy biochemical recurrence (INDICATE): ECOG-ACRIN EA8191. J Clin Oncol. 2023; 41:Number 6_suppl.
Radium-223 in 2024 and Beyond: Resurrected and Disrupting the First-Line mCRPC Treatment Landscape
Introduction
Radium-223 dichloride (radium-223) is a targeted alpha emitter that selectively binds to areas of increased bone turnover in bone metastases and emits high-energy alpha particles of short-range (<100 μm). Radium-223 acts as a bone-seeking calcium mimetic and binds into newly formed bone stroma, especially within the microenvironment of osteoblastic or sclerotic metastases. Double-stranded DNA breaks result secondary to the high-energy alpha-particle radiation.- Written by: Rashid Sayyid MD, MSc and Zachary Klaassen, MD, MSc
- References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369(3):213-223.
- Parsons MW, Tward JD. Utilization of Radium-223 in Metastatic Prostate Cancer Patients and Novel Prognostic Indicators in Radium-223 Patients. Int J Rad Oncol Biol Phys. 2020;108(3):e899.
- Ahmadzadehfar H, Azgomi K, Hauser S, et al. 68Ga-PSMA-11 PET as a Gatekeeper for the treatment of metastatic prostate cancer with 223Ra: Proof of Concept. J Nucl Med. 2017; 58(3):438-444.
- Rahbar K, Essler M, Eiber M, et al. Safety and Effectiveness of Lutetium-177-Prostate Specific Membrane Antigen (177Lu-PSMA) Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) Previously Treated with Radium-223 (223Ra): The RALU Study. J Nucl Med. 2023; 64(12):1925-1931.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368(2):138-148.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371(5):424-433.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20(3):408-419.
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med. 2023; 389(16):1453-1465.
- Leith A, Ribbands A, Kim J, et al. Impact of next-generation hormonal agents on treatment patterns among patients with metastatic hormone-sensitive prostate cancer: a real-world study from the United States, five European countries and Japan. BMC Urol. 2022; 22(1):33.
ESMO 2024 Quick Take Insights: Prostate Cancer
Introduction
At the ESMO 2024 annual meeting in Barcelona, Spain, the results of numerous practice-changing trials were presented, including those from three highly-anticipated clinical trials in the advanced prostate cancer space:
- Written by: Zachary Klaassen, MD, MSc, Wellstar MCG Health, Augusta, GA and Rashid Sayyid, MD, MSc, University of Southern California, Los Angeles, CA
- References:
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022; 386(12):1132-1142.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368(2):138-148.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371(5):424-433.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20(3):408-419.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021; 385(12):1091-1103.
ESMO 2024 Quick Take Insights: A Focus on PEACE-3
ESMO 2024 Quick Take Insights: A Focus on PEACE-3
The ESMO 2024 annual meeting in Barcelona, Spain, featured many practice-changing trials and hypothesis-generating data in genitourinary cancer. One of the most highly anticipated trials in the metastatic castration-resistant prostate cancer (mCRPC) disease space was the phase III PEACE-3 trial – the focus of this ESMO 2024 Quick Take Insights.
- Written by: Zachary Klaassen, MD, MSc Wellstar MCG Health, Augusta, GA & Rashid Sayyid, MD, MSc, University of Southern California, Los Angeles, CA
- References:
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368(2):138-148.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371(5):424-433.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Rahbar K, Essler M, Pabst KM, et al. Safety and Survival Outcomes of 177Lu-Prostate-Specific Membrane Antigen Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer with Prior 223Ra Treatment: The RALU Study. J Nucl Med. 2023 Apr;64(4):574-578.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20(3):408-419.
ESMO 2024 Quick Take Insights: A Focus on ARANOTE
- Written by: Zachary Klaassen, MD, MSc Wellstar MCG Health, Augusta, GA & Rashid Sayyid, MD, MSc, University of Southern California, Los Angeles, CA
- References:
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022; 386(12):1132-1142.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019 Nov 10;37(32):2974-2986.
ESMO 2024 Quick Take Insights: A Focus on SPLASH
ESMO 2024 Quick Take Insights: A Focus on SPLASH
The ESMO 2024 annual meeting in Barcelona, Spain, featured many practice-changing trials and hypothesis-generating data in genitourinary cancer. One of the most highly anticipated trials in the metastatic castration-resistant prostate cancer (mCRPC) disease space was the phase III SPLASH trial – the focus of this ESMO 2024 Quick Take Insights.
- Written by: Zachary Klaassen, MD, MSc Wellstar MCG Health, Augusta, GA & Rashid Sayyid, MD, MSc, University of Southern California, Los Angeles, CA
- References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021; 385(12):1091-1103.
- Morris MJ, Castellano D, Herrmann K, et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naïve patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomized, controlled trial. Lancet 2024 Sep 28;404(10459):1227-1239.
Treatment Intensification for High-Risk Biochemically Recurrent M0 HSPC: Quality of Life and Sexual Function Considerations
Introduction
In November 2023, the United States Food and Drug Administration (FDA) approved enzalutamide, with or without concurrent leuprolide therapy, for non-metastatic hormone-sensitive prostate cancer (M0 HSPC) patients with biochemical recurrence at high risk for metastasis.1 This drug approval followed the publication of the EMBARK trial, a randomized phase III trial of biochemically recurrent patients who had high-risk disease, defined by a PSA doubling time (PSADT) ≤9 months and a PSA level of ≥2 ng/mL above nadir following radiation therapy or ≥1 ng/mL after radical prostatectomy, with or without postoperative radiation therapy. Patients in this trial were randomized 1:1:1 to combination enzalutamide + leuprolide, leuprolide monotherapy, or enzalutamide monotherapy. This trial met its primary endpoint of improved 5-year metastasis-free survival with the combination of enzalutamide + leuprolide (87.3% versus 71.4% for leuprolide alone; HR 0.42, p < 0.001). Similarly, enzalutamide monotherapy was associated with superior 5-year metastasis-free survival rates, compared to leuprolide monotherapy (80% versus 71%; HR 0.63, p = 0.005).2While both enzalutamide monotherapy and combination therapy with leuprolide have demonstrated promising efficacy outcomes for these high-risk patients, it is important to consider the safety profiles of these drugs and their impact on health-related quality of life measures. These patients have a prolonged natural history,3 and thus their survivorship care must remain of utmost importance.
In this Center of Excellence article, we will discuss the health-related quality of life effects of the different treatment arms of the EMBARK trial and highlight important functional outcomes considerations, particularly with regard to sexual function.
EMBARK
In the EMBARK trial, patient-reported outcomes were assessed at baseline and every 12 weeks thereafter until disease progression or completion of data gathering. For each patient-reported outcomes instrument, change from baseline (i.e., post-baseline value minus baseline value) was calculated for each assessment of a domain. Health-related quality of life was measured using Brief Pain Inventory–Short Form (BPI-SF), Functional Assessment of Cancer Therapy-Prostate (FACT-P), Quality of Life Questionnaire–Prostate 25 (QLQ-PR25), and European Quality of Life 5-Dimensions 5-Levels health questionnaire (EQ-5D-5L) scores.4At baseline, the questionnaire completion rates were ≥90% and remained high (≥85%) up to week 205. The baseline patient-reported outcome scores indicated low levels of pain and high health-related quality of life scores, suggesting that most patients were asymptomatic at baseline. However, sexual activity scores were low likely secondary to prior treatments received (50% radical prostatectomy and radiotherapy, 25% radical prostatectomy alone, 25% radiotherapy alone). Very few patients reported hormonal therapy-related symptoms at baseline, as only 31% had received prior hormones and none within the preceding nine months.
Overall, the patient-reported outcome scores remained stable throughout the trial for most patients in all groups, suggesting maintenance of the high baseline health-related quality of life and low baseline symptoms across all groups, with no clinically meaningful differences overall across the groups.
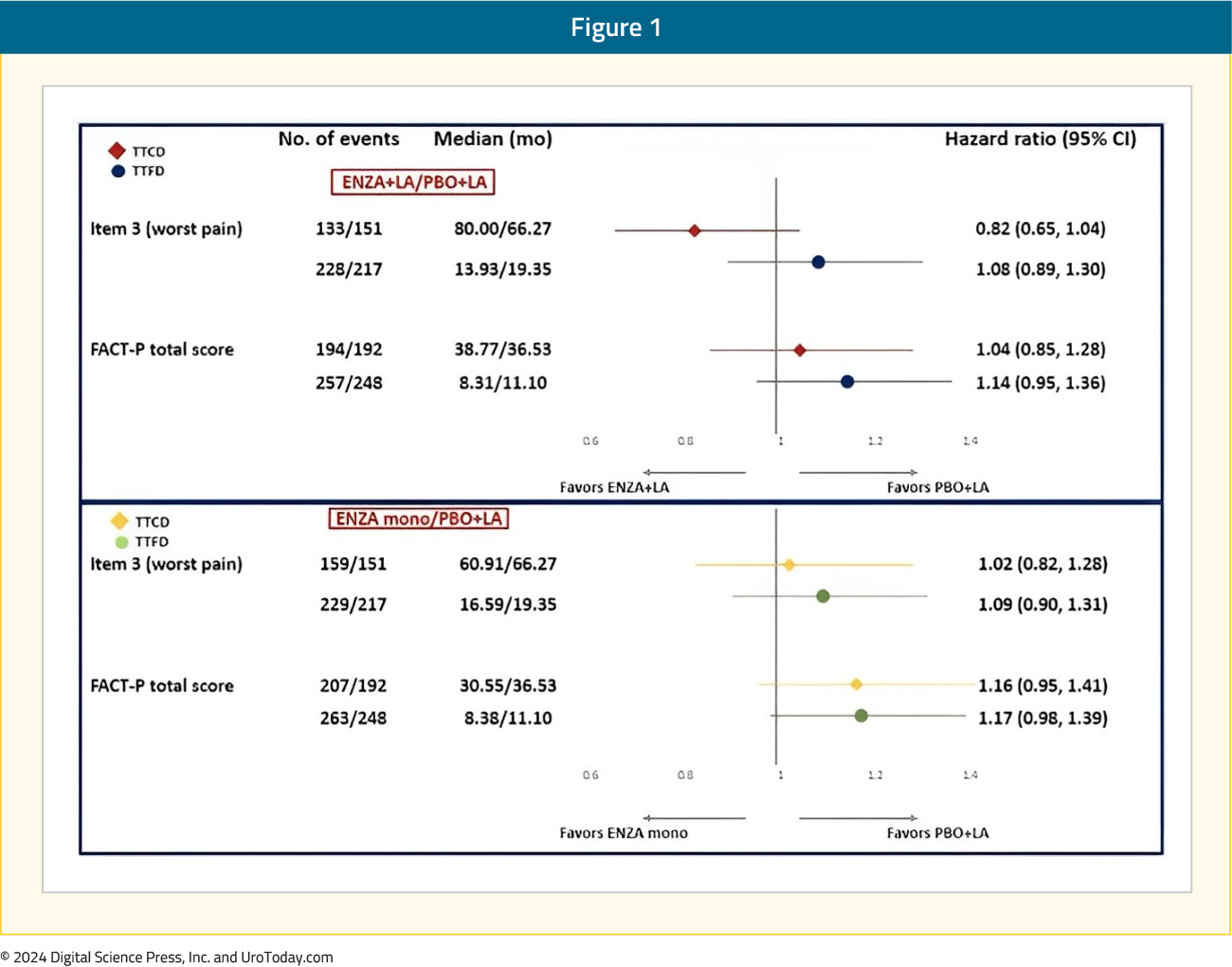
Key Outcomes: Worst Pain in Prior 24 Hours and Functional Status
There were no differences in either the time to first or confirmed clinically meaningful deteriorations in worst pain in the past 24 hours (BPI-SF item 3) or in functional status measured using FACT-P total score:
FACT-P Subdomains
The FACT-P total score longitudinally worsened in all three arms, with no clinically meaningful differences between the three arms (leuprolide alone: -3.9; enzalutamide combination: -5.7; enzalutamide monotherapy: -6.4):
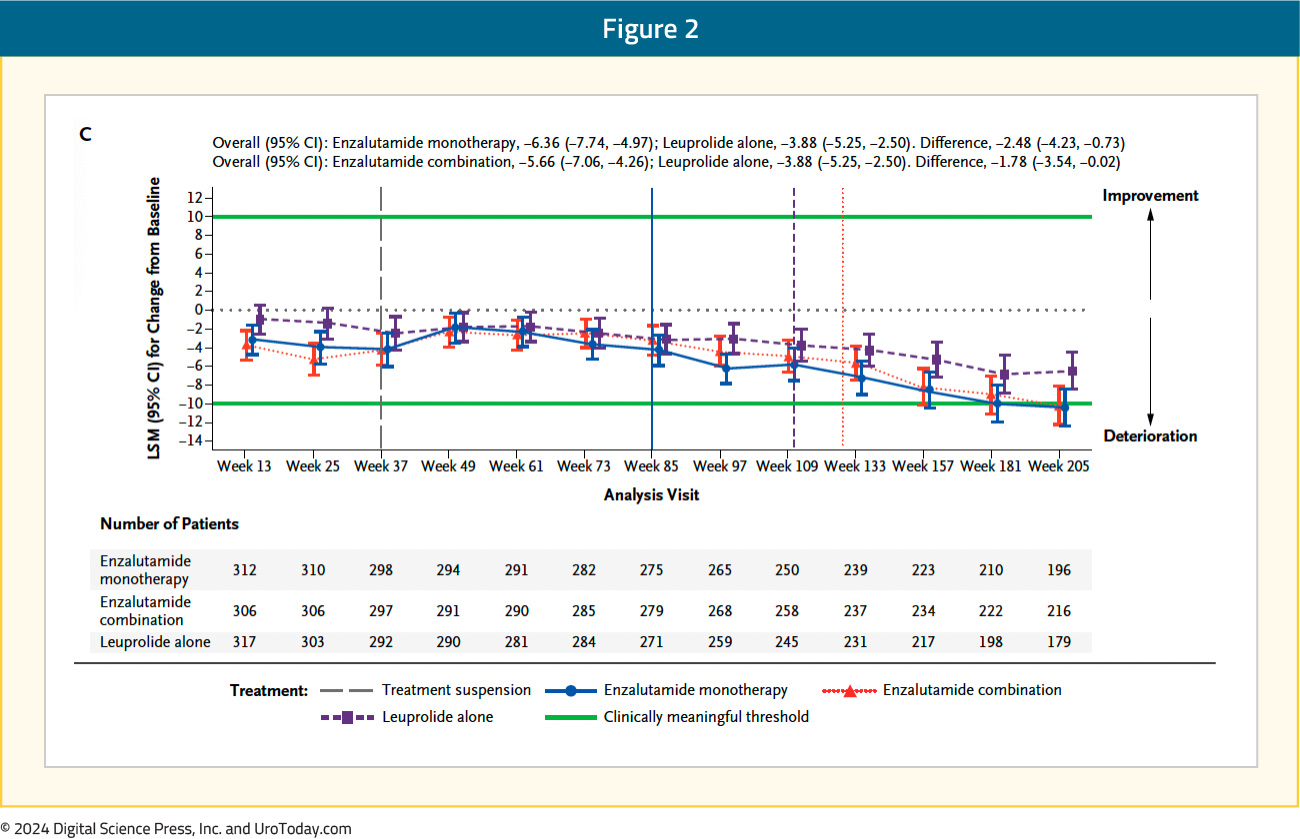
Within the individual FACT-P domains, patients in both the enzalutamide combination and monotherapy arms had significantly shorter (i.e., worse) time to confirm clinically meaningful deterioration in the physical well-being score, compared to leuprolide alone. Time to confirmed clinically meaningful deterioration in physical well-being score was 49.8 months with leuprolide, compared to 24.8 and 27.6 months for enzalutamide combination and monotherapy, respectively (HRs 1.41 and 1.35, respectively).
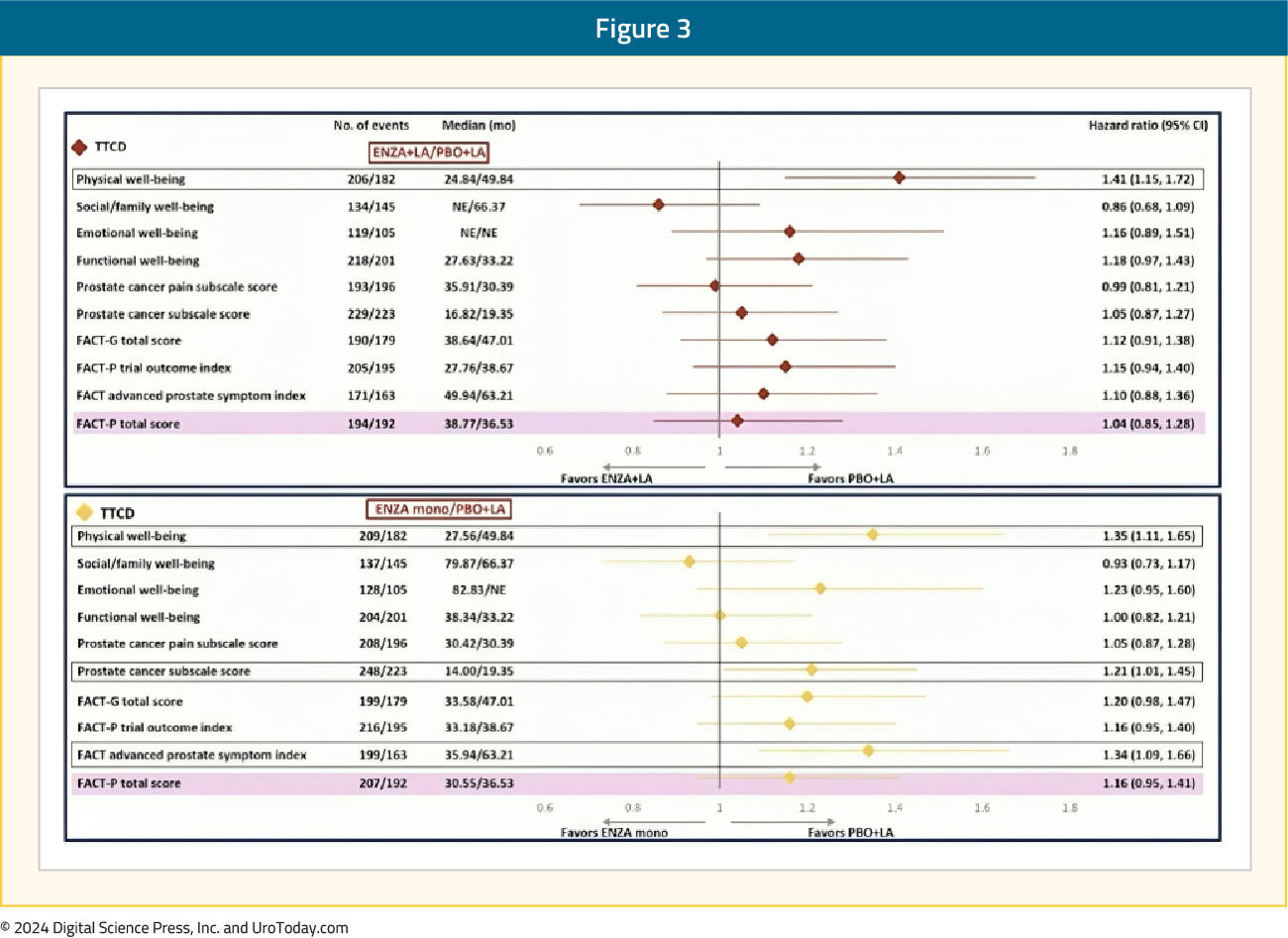
Patients in the enzalutamide monotherapy arm had a significantly worse median time to first clinically meaningful deterioration in the prostate cancer subscale score (HR 1.21) and advanced prostate symptom index (HR 1.34), compared to leuprolide-only therapy. There were otherwise no significant differences observed in the times to first or confirmed clinically meaningful deteriorations for the other FACT-P domains, including social/family, emotional, and functional well-being and prostate cancer pain domains:
Sexual Function and Hormonal Symptoms
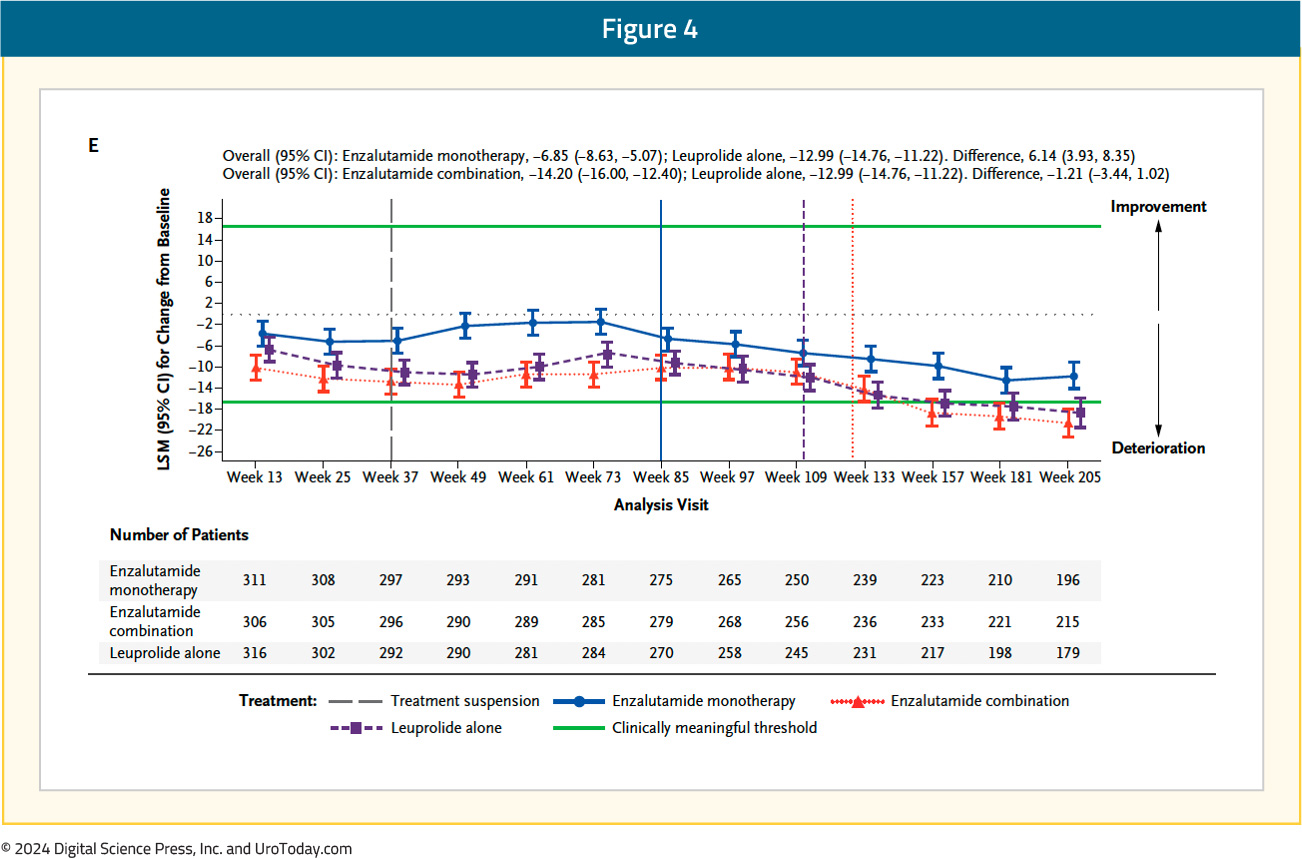
With regards to QLQ-PR25, the time to confirmed clinically meaningful deterioration of composite sexual activity was significantly longer (i.e., better) with enzalutamide monotherapy, compared to leuprolide-only therapy (median: 5.6 versus 3 months; HR 0.76, 95% CI 0.62–0.94). The longitudinal changes in sexual function are summarized below, with enzalutamide monotherapy denoted below in blue:
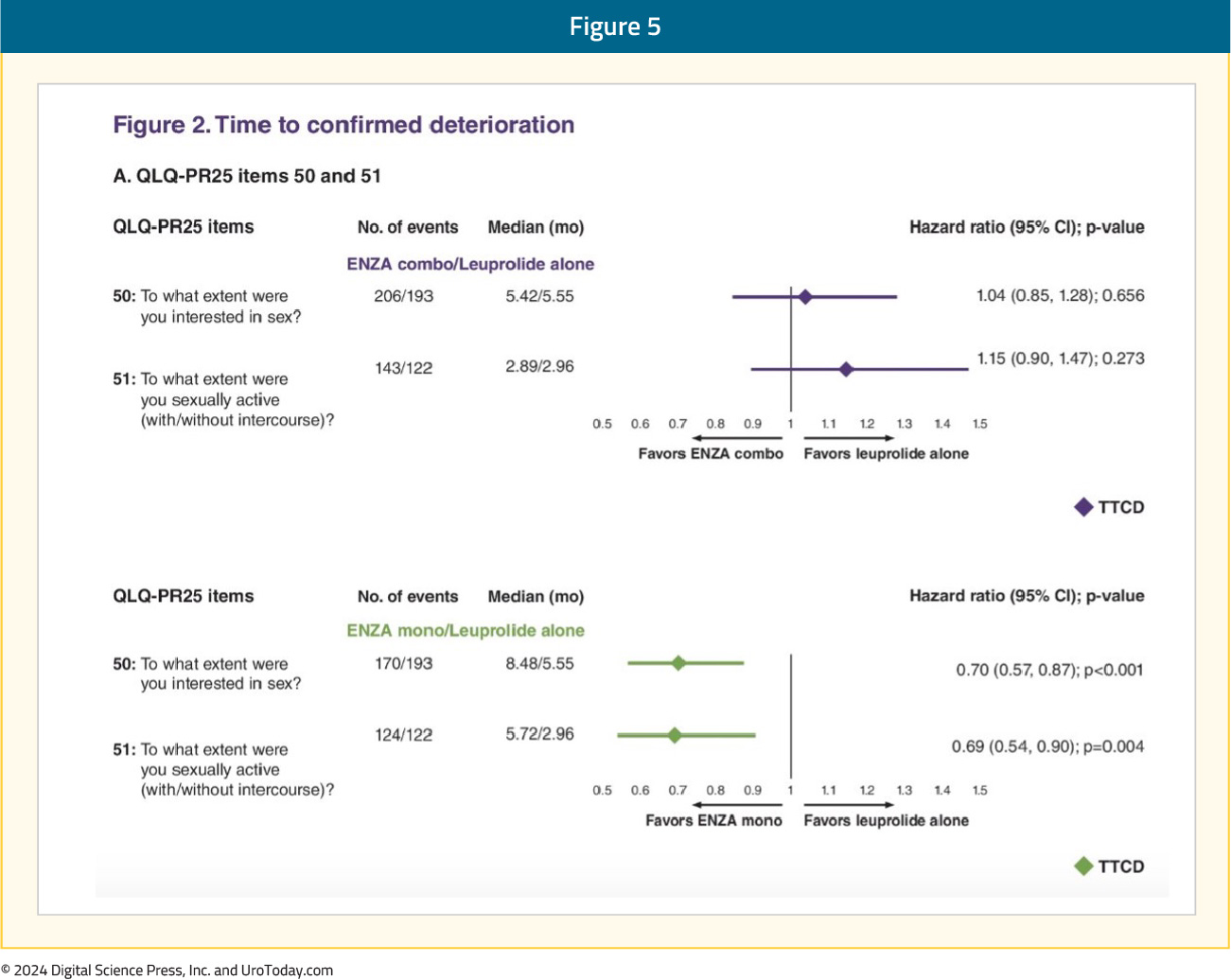
An individual item-level analysis of sexual function was presented at ASCO GU 2024 and demonstrated that enzalutamide monotherapy, compared to leuprolide monotherapy, was associated with significantly longer times to deterioration of sexual interest and sexual activity. Patients in the enzalutamide monotherapy arm had significantly higher satisfaction with their sexual life and ability to achieve and maintain erections. In the comparison of enzalutamide combination to leuprolide monotherapy, patients in the enzalutamide combination arm had significantly worse ability to achieve and maintain erections:5
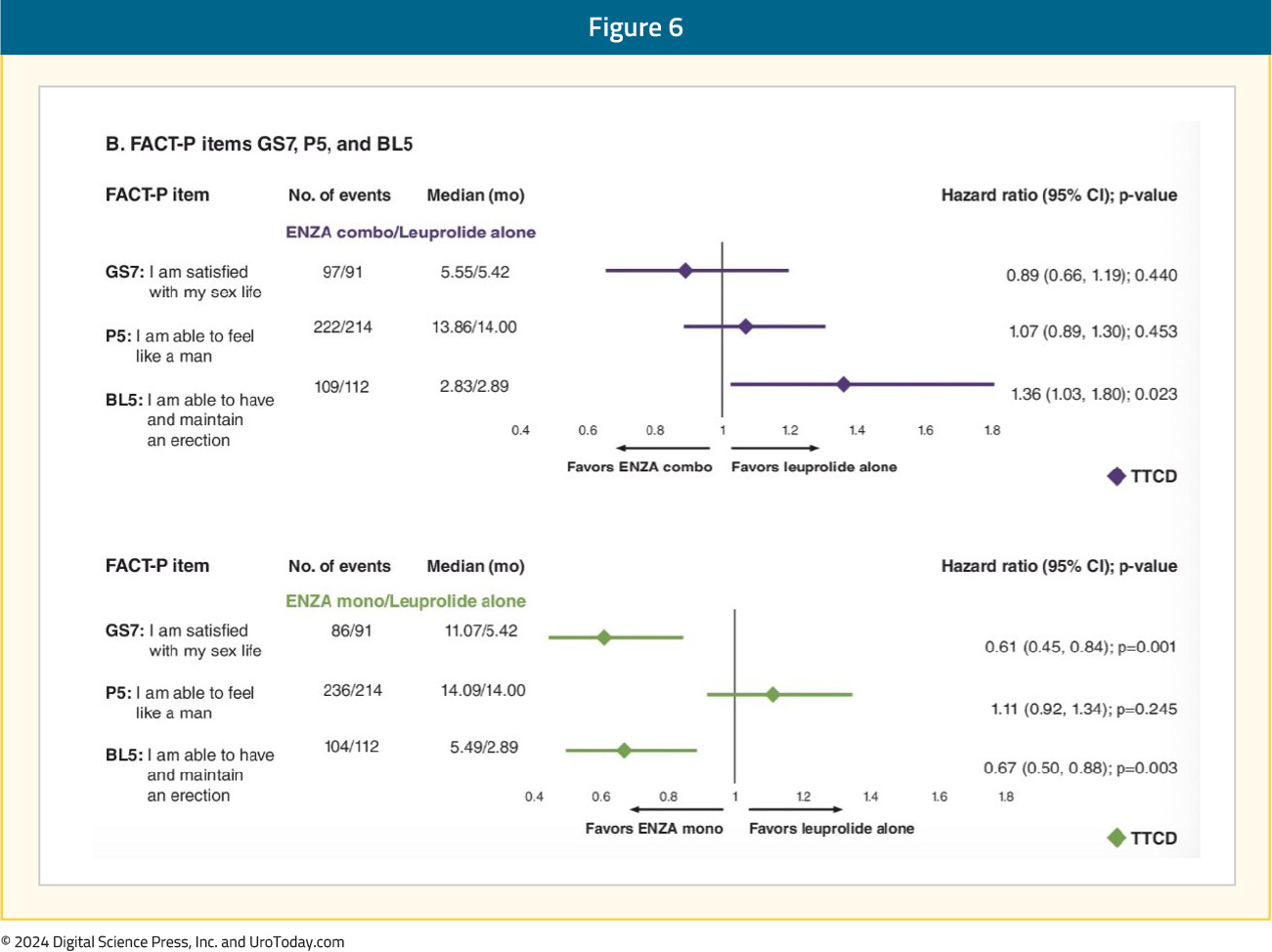
The time to confirmed clinically meaningful deterioration in hormonal treatment-related symptoms with enzalutamide combination was significantly worse compared to leuprolide alone (HR 1.19, 95% CI 1.01–1.40), although there were minimal differences in the median times to deterioration (2.86 versus 2.89 months):
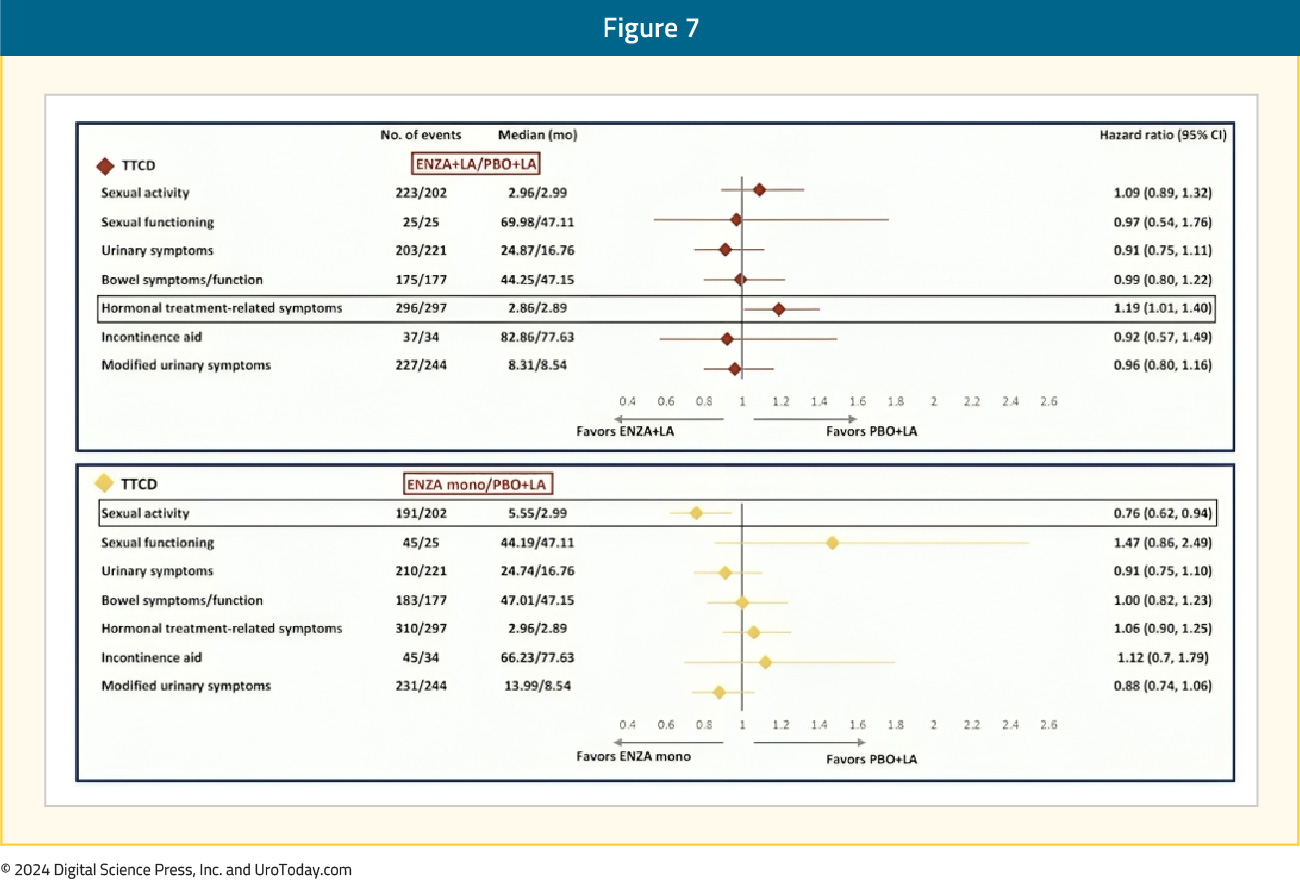
Safety
No new safety signals were observed with the combination of enzalutamide and leuprolide. Grade ≥3 adverse events were observed in 43-50% of patients across the three arms. The most common adverse events (occurring in ≥10% of patients) in the enzalutamide combination group and the leuprolide-alone group were hot flashes (57–69%) and fatigue (33–43%). The most common adverse events in the monotherapy group were gynecomastia (45% versus 8–9% in the two other arms), hot flashes (22%), and fatigue (47%). Notable adverse events in the enzalutamide monotherapy group were nipple pain (15% versus 1–3% in the other two arms) and breast tenderness (14% versus 1% in the other two arms). Fractures were more common in the enzalutamide combination group (18.4%), compared to the leuprolide-alone group (14%) and the enzalutamide monotherapy group (11%).
Treatment was discontinued due to adverse events in 21% of patients in the enzalutamide combination group, 10% of patients in the leuprolide-alone group, and 18% of patients in the enzalutamide monotherapy arm. The most common adverse event leading to discontinuation in all three arms was fatigue.
In a post-hoc analysis of outcomes stratified by age presented at ESMO 2024, serious adverse events were more common in patients aged ≥70 years vs <70 years for enzalutamide + leuprolide (45.0% vs 25.3%), leuprolide alone (36.2% vs 27.1%), and enzalutamide monotherapy (43.9% vs 30.4%). For clustered treatment emergent adverse events of special interest of any grade, musculoskeletal events, fatigue, and hypertension were common across all age and treatment groups.
Between-arms differences are summarized below (all comparisons are to leuprolide-only therapy):
- Physical well-being score: Significantly worse time to confirmed clinically meaningful deterioration of physical well-being score for both enzalutamide combination and monotherapy arms (leuprolide: 50 months; enzalutamide combination/monotherapy: 25–28 months). These scores likely reflect the higher incidence of fatigue with enzalutamide combination (43%) or monotherapy (47%), versus leuprolide-only therapy (33%).
- Prostate cancer subscale score and advanced prostate symptom index: Worse for enzalutamide monotherapy patients
- Social/family, emotional, and functional well-being and prostate cancer pain FACT-P domains: No differences between the arms
- Sexual function: Deteriorates in all three arms. Significantly longer time to confirmed clinically meaningful deterioration of composite sexual activity with enzalutamide monotherapy (5.6 versus 3 months). Patients treated with enzalutamide monotherapy reported superior sexual interest and activity and had higher satisfaction with their sexual life and ability to achieve and maintain erections. Enzalutamide combination therapy patients had significantly worse ability to achieve and maintain erections.
- Hormonal treatment-related symptoms: Enzalutamide combination patients had a significantly worse time to confirmed clinically meaningful deterioration in hormonal treatment-related symptoms
- Notable adverse events:
- Hot flashes: More common in the enzalutamide + leuprolide and leuprolide-only arms (57–69% versus 22% for enzalutamide monotherapy)
- Gynecomastia nipple pain, and breast tenderness: More common with enzalutamide monotherapy
- Gynecomastia: 45% versus 8–9%
- Nipple pain: 15% versus 1–3%
- Breast tenderness: 14% versus 1%
Summary and Take-Home Messages
In this analysis of patient-reported outcomes from the EMBARK trial, the key take-home message is that the majority of trial patients maintained overall stable health-related quality-of-life outcomes throughout the study. However, there are notable differences between the study arms with respect to their relative impact on specific quality-of-life domains. A comprehensive understanding of these differences is key to engaging these patients in a shared decision-making process to strike a balance between maximizing oncologic benefits while maintaining quality-of-life measures that are most significant for the individual patients.Published October 2024
- Written by: Rashid Sayyid, MD, MSc, Robotic Urological Oncology Fellow, Department of Surgery, Section of Urology, University of Southern California, Los Angeles, CA & Zachary Klaassen, MD, MSc, Assistant Professor Surgery/Urology at the Medical College of Georgia at Augusta University, Wellstar MCG Health, Augusta, GA
- References:
- FDA approves enzalutamide for non-metastatic castration-sensitive prostate cancer with biochemical recurrence.
- Freedland SJ, de Almeida Luiz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med. 2023; 389(16):1453–65.
- Pound CR, Partin AW, Eisenberger MA, et al. Natural History of Progression After PSA Elevation Following Radical Prostatectomy. JAMA. 1999; 28(17):1591–7.
- Freedland SJ, Gleave M, De Giorgi U, et al. Enzalutamide and Quality of Life in Biochemically Recurrent Prostate Cancer. NEJM Evidence. 2023; 2(12).
- Freedland SJ, Mulhall J, Gleave M, et al. EMBARK Post Hoc Analysis of Sexual Activity Patient-Reported Outcome Measures. J Clin Oncol. 2024; 42:Number 4_suppl.
Treatment Intensification for High-Risk Biochemically Recurrent M0 HSPC: ‘EMBARK’ing on a Novel Treatment Paradigm
Introduction
Historically, the recommended management for biochemically recurrent prostate cancer patients following maximal pelvic therapy with no metastasis on conventional imaging has been disease monitoring, with androgen deprivation therapy (ADT) reserved for metastatic disease development. Studies of the natural history of such patients have demonstrated that the median time to development of metastases is approximately eight years, with 82% of patients remaining free of conventional imaging-defined metastasis 15 years following surgery.1- Written by: Rashid K. Sayyid, MD, MSc, University of Southern California Los Angeles, CA & Zachary Klaassen, MD MSc Wellstar MCG Health Augusta, GA
- References:
- Pound CR, Partin AW, Eisenberger MA, et al. Natural History of Progression After PSA Elevation Following Radical Prostatectomy. JAMA. 1999; 28(17):1591–7.
- Freedland SJ, Humphreys EB, Mangold LA, et al. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007; 25(13):1765–71.
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005; 294(4):433–9.
- Schaeffer EM, Srinivas S, Adra N, et al. NCCN Guidelines® Insights: Prostate Cancer, Version 3.2024. J Natl Compr Canc Netw. 2024; 22(3):140–50.
- Duchesne GM, Woo H, Bassett JK, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016; 17(6):727–37.
- Freedland SJ, de Almeida Luiz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med. 2023; 389(16):1453–65.
- Aggarwal R, Heller G, Hillman DW, et al. PRESTO: A Phase III, Open-Label Study of Intensification of Androgen Blockade in Patients With High-Risk Biochemically Relapsed Castration-Sensitive Prostate Cancer (AFT-19). J Clin Oncol. 2024; 42(10):1114–23.
- FDA approves enzalutamide for non-metastatic castration-sensitive prostate cancer with biochemical recurrence. Accessed on August 30, 2024.
Metastasis-Directed Therapy in Oligometastatic Hormone-sensitive Prostate Cancer: New Frontiers in Advanced Prostate Cancer
Introduction
Classical theories of metastasis have followed ‘the seed and soil’ hypothesis, the Halstedian model, which proposes an orderly spread of disease from local to distant sites, with the presumption that cancer is an inherently systemic process even in the earliest cases. More contemporary spectrum theories now suggest that the propensity for distant spread exists along a ‘metastatic continuum’. Tumors with limited metastatic potential (i.e., oligometastases) represent a unique subset along this spectrum that could be potentially cured with local ablative therapy (i.e., metastasis-directed therapy [MDT]). This concept is not unique to prostate cancer and has been evaluated in other disease sites including non-small cell lung, colorectal, esophageal, and breast cancers.1
The majority of the evidence for MDT in the prostate cancer space, to date, has been for patients with recurrent, oligometastatic hormone sensitive prostate cancer. MDT has been evaluated both in lieu of, to avoid/delay the use of systemic therapy, and in combination with systemic therapy to potentially improve efficacy outcomes. In this Center of Excellence article, we discuss the evidence and practical applications for MDT in the recurrent oligometastatic hormone sensitive setting.
Trials of MDT versus Observation/Surveillance for Recurrent Oligometastatic Prostate Cancer
To date, three prospective phase II trials have compared MDT to observation for patients with recurrent oligometastatic hormone sensitive prostate cancer.
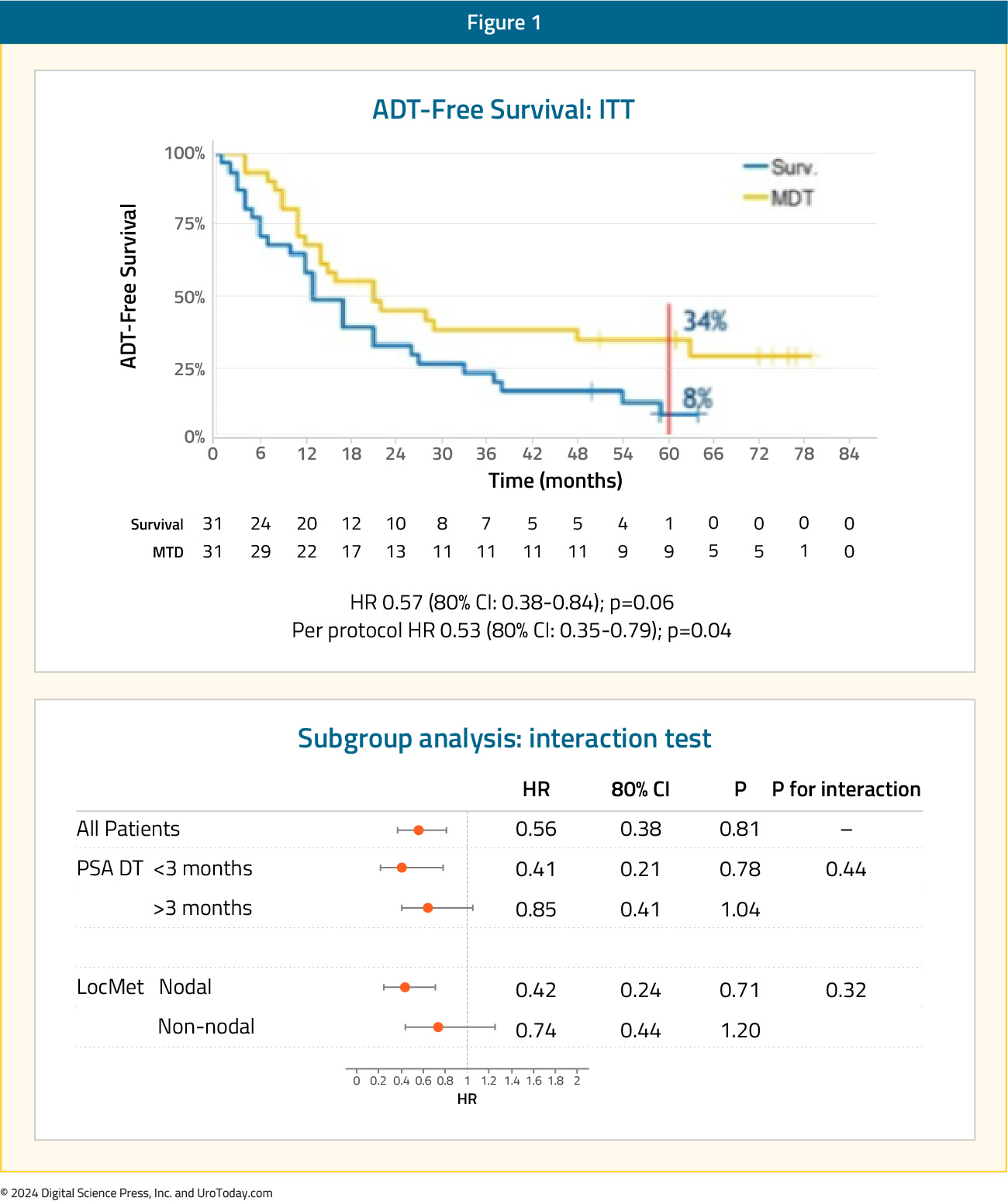
STOMPThe STOMP trial was a multicenter, randomized phase II trial that prospectively evaluated the effects of MDT for eugonadal men with evidence of oligometastatic disease on choline PET/CT (up to three extracranial sites) who had received prior treatment with curative intent and had evidence of biochemical recurrence. Between 2012 and 2015, 62 patients were randomized 1:1 to either surveillance or MDT, consisting of stereotactic body radiotherapy (SBRT) or metastasectomy. The primary endpoint was time to initiation of ADT (i.e., ADT-free survival). ADT was initiated for symptoms, progression beyond three metastases, or local progression of known metastatic disease
After a median follow up of 5.3 years, the five-year ADT-free survival was 8% in the surveillance arm compared to 34% for the MDT group (HR: 0.57, 95% CI: 0.38–0.84, log-rank p = 0.06). No differences were seen between groups when stratified by nodal versus non-nodal metastases:
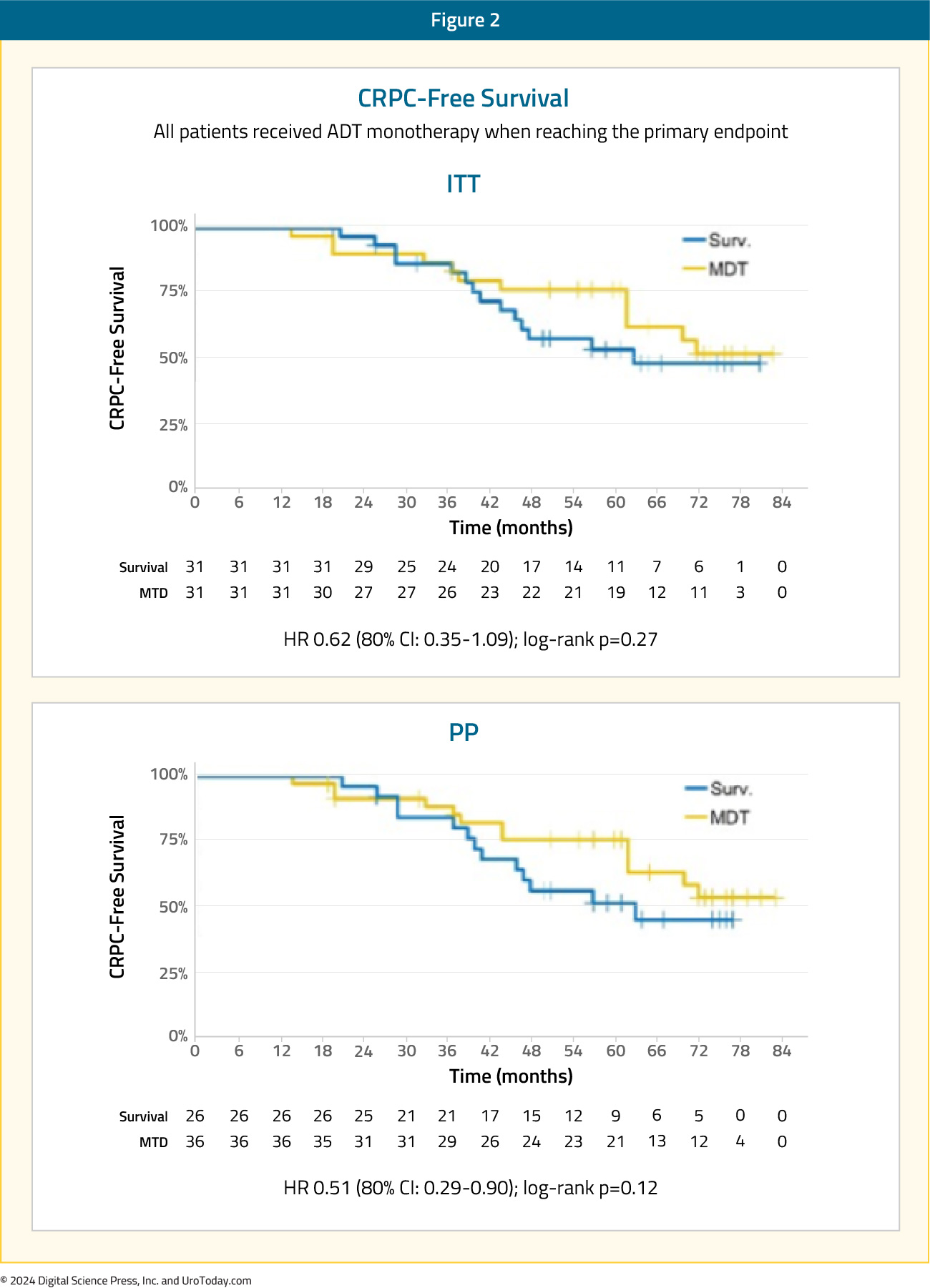
The secondary endpoint of 5-year CRPC-free survival was 53% in subjects under surveillance and 76% in those receiving MDT (HR 0.62, 80% CI: 0.35-1.09):2
The ORIOLE trial was a randomized phase II trial of men with recurrent oligometastatic hormone-sensitive prostate cancer (up to three sites). Between 2016 and 2018, 80 men were screened, of which 54 men had 1 to 3 metastases detectable by conventional imaging and had not received ADT within 6 months of enrollment or 3 or more years total. These 54 men were randomized in a 2:1 fashion to receive SBRT or observation. The primary outcome was disease progression at 6 months, defined by a serum PSA increase, radiographic progression on conventional imaging, symptomatic progression, ADT initiation for any reason, or death.
After a median follow-up of 19 months, disease progression at six months occurred in 19% of patients in the SBRT arm versus 61% of patients in the observation arm (p = 0.005). Patients in the SBRT treatment arm had superior median progression-free survival rates (median: not reached versus 5.8 months; HR: 0.30; 95% CI: 0.11–0.81; p = 0.002):
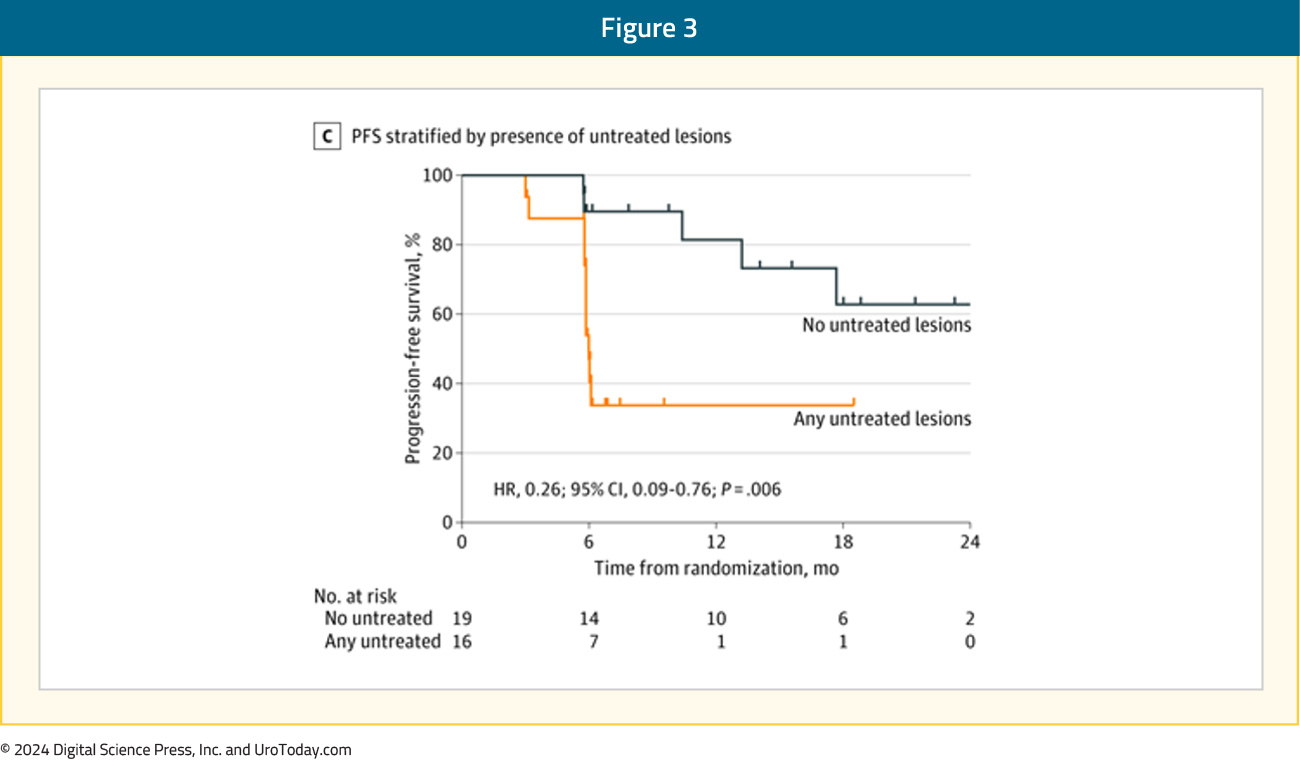
Secondary to the blinding of the investigative team to the PSMA-targeted PET data during treatment planning, 16 of 36 men treated with SBRT had baseline PET-avid lesions that were not included in the treatment fields. The proportion of men with no untreated lesions with progression at 6 months was 1 of 19 (5%) compared with 6 of 16 (38%) for those with any untreated lesions (p = 0.03). The median progression free survival was unreached among men with no untreated lesions vs 11.8 months among participants with any untreated lesions (HR, 0.26; 95% CI, 0.09-0.76; p = 0.006):3
As such, this was among the first data to suggest the importance of treating all PSMA PET-visible lesions for maximal oncologic benefit in the oligometastatic mHSPC space.
ORIOLE + STOMP: Pooled DataIn 2022, pooled data from the ORIOLE and STOMP trials demonstrated that MDT improves progression free survival from 5.9 months to 11.9 months (HR: 0.44, p<0.001), however without any significant improvements seen in radiographic progression-free survival, time to castration-resistant disease, or overall survival:4

SABR-COMET was a randomized, open-label phase II study of patients with oligometastatic disease (up to five sites) between February 2012 and August 2016. This trial was not restricted to patients with prostate cancer and also included lung, breast, and colorectal cancer patients. Of the 99 patients in this trial, 18 (18.2%) had prostate cancer. After stratifying by the number of metastases (1–3 versus 4–5), patients were randomized in a 1:2 fashion to receive either palliative standard of care alone or standard of care plus SBRT.
At a median follow-up of 5.7 years, the primary outcome of overall survival was superior for SBRT-treated patients. The 8-year overall survival rates were 27% and 14% in the intervention and control arms, respectively (HR: 0.50, 95% CI: 0.30–0.84, p = 0.008). The 8-year progression-free survival estimates were 21% and 0%, respectively (HR: 0.45, 95% CI: 0.28–0.72, p < 0.001):

The rates of grade ≥2 acute or late toxic effects were 30% versus 9% (p = 0.019), and the FACT-G quality of life scores declined over time in both arms, but with no differences in quality-of-life scores between the study arms.5
Combination of MDT + Systemic Therapy for Recurrent Oligometastatic Prostate Cancer
EXTENDThe EXTEND trial was a single center, phase II randomized controlled trial of 87 oligorecurrent men, mostly with mHSPC (>90%), who were randomized 1:1 to intermittent hormone therapy +/- MDT (definitive radiation therapy to all sites of disease). All patients had ≤5 metastases, as defined by conventional imaging (75%) or fluciclovine PET/CT (25%). A planned break in hormone therapy occurred 6 months after enrollment, after which hormone therapy was withheld until progression. At a median follow-up of 22 months, progression free survival was improved in the combined therapy arm (HR: 0.25, 95% CI: 0.12 – 0.55, p < 0.001). Significantly, ‘eugonadal’ progression free survival was also improved with this combination approach (HR: 0.32, p = 0.03):6
SATURN is a phase II trial of 28 men with oligorecurrent extra-pelvic metastases on PSMA-PET/CT following initial treatment with radical prostatectomy. Patients were treated with 6 months of ‘androgen annihilation therapy’, defined as leuprolide + abiraterone acetate/prednisone + apalutamide. After the 1st month of this systemic therapy, patients received SBRT to all metastases with or without radiotherapy directed to the prostate bed and pelvic lymph nodes. Results of the primary endpoint, the percentage of patients who maintained PSA <0.05 ng/mL six months after testosterone recovery to ≥150 ng/dL, were presented at ASCO GU 2024. Overall, 50% of patients maintained a PSA <0.05 ng/mL six months after testosterone recovery. After a median follow-up of 20 months, the median progression free survival was 19.3 months. Moreover, 81% of patients recovered eugonadal testosterone levels, at a median of 9.4 months from the start of systemic therapy, and the median eugonadal progression free survival was 11.4 months. Grade 3 adverse events related to androgen therapy were observed in 21% of patients, and SBRT had 7.7% grade 2 and no grade 3 toxicity.9
Key Ongoing Trials of MDT + Systemic TherapyThere are several important ongoing trials combining systemic therapy with the site specific control offered by MDT. The ADOPT trial is an ongoing phase III trial that is randomizing 280 patients with evidence of recurrent oligometastatic disease (≤4 lesions) on PSMA-PET/CT 1:1 to either MDT (radiotherapy) alone or MDT + 6 months of ADT. The primary endpoint of this trial is 30 month metastases progression free survival, with key secondary endpoints including overall survival, adverse events, and quality of life outcomes:7

NRG GU011 (PROMETHEAN) is a randomized phase II trial of SBRT with or without relugolix for early PET-detected recurrent oligometastatic prostate cancer. Eligible patients are those with biochemical recurrence following prior curative intent radiation or surgery for localized prostate cancer, PSA < 10 ng/mL, negative conventional imaging, and 1–5 PET-visible metastases (≥1 extra-pelvic). The primary endpoint is conventional imaging-based radiographic progression free survival. Key secondary endpoints include PET-based radiographic progression free survival, overall survival, and quality-of-life outcome measures:

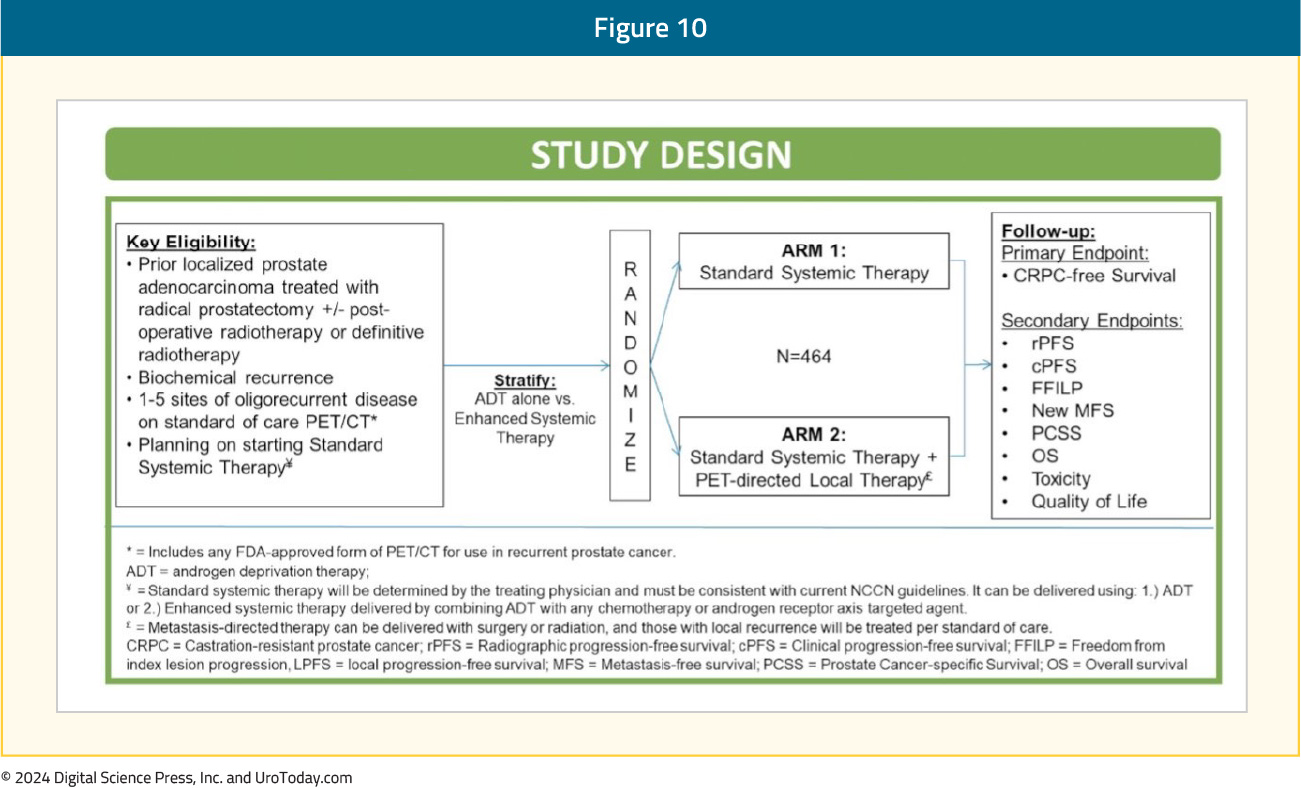
The VA STARPORT study is a phase II/III randomized trial that evaluates the value of adding PET-directed local therapy to standard systemic therapy for biochemically recurrent patients with evidence of ≤5 metastatic lesions on PSMA-PET/CT. PET-directed local therapy is defined as surgery or radiation to all visible metastases and any prostate/prostatectomy bed local recurrences. Systemic therapy will be administered indefinitely, consistent with current guidelines. The primary study endpoint is castrate-resistant prostate cancer-free survival. Key secondary endpoints include clinical and radiographic progression free survival, overall survival, toxicity, and quality of life outcomes:
PERSIAN is a randomized phase II trial of recurrent oligometastatic, hormone-sensitive patients (<5 non-visceral lesions). Eligible patients will undergo 1:1 randomization to apalutamide + ADT +/- SBRT to all visible lesions. The primary outcome is 6-month complete biochemical response. The trial design and key secondary endpoints are illustrated below:

The POSTCARD GETUG P13 is a French randomized phase II trial that is randomizing patients with recurrent oligometastatic disease following primary local therapy with curative intent to either SBRT alone (to all visible metastases) or SBRT + durvalumab. Oligometastasis is defined as ≤5 bone or lymph node metastases on 68Ga-PSMA PET/CT or ≤3 bone or lymph node metastases on 18F-choline PET/CT. The primary endpoint is two-year progression-free survival, with key secondary endpoints of androgen deprivation therapy free survival, quality of life, toxicity, prostate cancer specific survival, overall survival, and immune response.8
Incorporating PSMA-PET/CT into the MDT Paradigm
The next ‘frontier’ of MDT trials is incorporating PSMA-PET/CT for staging oligometastatic patients and determining eligibility for MDT. Given the increased sensitivity of PSMA-PET/CT compared to conventional imaging,10 it is likely that this may lead to a ‘stage migration’ phenomenon, where future cohorts of oligometastatic prostate cancer patients, matched for the same number of metastatic lesions, have more favorable prognoses. However, there has been a shift away from defining the ‘upper limit’ of oligometastatic disease by the number of metastatic lesions. A recent survey of participants from the ESTRO-ASTRO consensus conference demonstrated that the majority of respondents concur that the ‘upper limit’ of oligometastatic disease should be defined by the ability to safely deliver curative intent metastasis-directed radiotherapy and not by the number of metastatic lesions.
Existing evidence suggests that PSMA-PET/CT provides additional information in patients with evidence of recurrent oligometastatic disease on conventional imaging. In the ORIOLE trial, as highlighted above, pre-treatment 18F-DCFPyL-PET/CT was performed in all patients assigned to the MDT arm (n = 36). Of the 36 patients treated with SBRT, 16 (44.4%) had baseline PET-avid lesions that were not included in the treatment fields. These patients had significantly worse 6-month progression rates of 38% compared to those without untreated lesions (5%; p = 0.03). Furthermore, those with untreated sites of disease had higher rates of new metastases (per conventional imaging) at 6 months (62.5% versus 15.8%, p = 0.006), and worse median distant metastasis-free survival of 6 versus 29 months (HR: 0.19; 95% CI: 0.07–0.54, p < 0.001). These results highlight the importance of targeting all sites of disease, and the value of PSMA PET/CT for defining this with increased sensitivity compared to conventional imaging.3
A single arm phase II study evaluated the role of MDT (SBRT or surgery) in 37 patients with a rising PSA (0.4–3 ng/ml) following maximal local therapy (radical prostatectomy + adjuvant/salvage radiotherapy) who had not received prior salvage hormonal therapy and had negative conventional imaging, but evidence of oligometastasis on 18F-DCFPyL PET-MR/CT. Ten and 27 patients underwent surgery and SBRT, respectively. At a median follow-up of 16 months, the overall response rate was 60%, including 22% who had biochemical ‘no evidence of disease. One (2.7%) grade 3 toxicity (intra-operative ureteric injury) was observed.11
MDT for De Novo Oligometastatic Hormone-sensitive Prostate Cancer
There are numerous ongoing trials evaluating the role of MDT in combination with systemic therapy in the de novo oligometastatic hormone-sensitive setting. In this section, we will highlight key trials in this space.
SOLAR (NCT03298087) is a single arm phase II trial of 28 patients with de novo M1a/b disease and 1–5 radiographically visible M1 lesions (majority detected via PSMA PET-CT) who all underwent radical local treatment, intensified systemic therapy for six months (leuprolide, abiraterone acetate with prednisone, apalutamide), and metastasis-directed SBRT. Radical local therapy was either radical prostatectomy (n = 12) with lymph node dissection and postoperative radiotherapy (for ≥pT3a, N1, or positive margins) or radical radiotherapy (n=12) directed to the prostate, seminal vesicles, and pelvic lymph nodes. The initial results of this trial were presented at GU ASCO 2024. At a median follow-up of 30 months, 62% of patients completed all planned systemic therapy without dose modification. Of the 22 patients with >6 months follow-up after testosterone recovery, 86% remained free of any progression, defined as an undetectable serum PSA after radical prostatectomy or <2 ng/ml after radical radiotherapy. Grade 2 and 3 toxicities for primary tumor therapy were 46% and 4%, respectively. There were no grade 2–3 toxicities relating to SBRT.12
PLATON (NCT03784755) is a two-arm phase II RCT exploring sequential treatment with ADT +/- chemotherapy followed by cytoreductive prostatectomy or external beam radiotherapy and then SBRT for oligometastases in 410 men. The comparator arm consists of systemic therapy alone, although low-volume men will be allowed to receive conventional local prostate radiotherapy. The primary study outcome is failure-free survival:

IP2-ATLANTA (NCT03763253) is a three-arm phase II randomized trial exploring sequential systemic, local, physical cytoreductive therapy and finally SBRT versus standard of care in 918 men with any-volume metastatic disease. All patients will receive doublet systemic therapy (ADT + docetaxel or enzalutamide or abiraterone). Patients in experimental arm 1 will receive minimally invasive ablative therapy +/- lymph node dissection, whereas those randomized to experimental arm 2 will receive local external beam radiotherapy +/- lymph nodes or radical prostatectomy +/- pelvic lymph node dissection. Patients in both experimental arms will further receive SBRT to oligometastases following receipt of of systemic and local treatment. Patients in the control arm will receive planned systemic therapy only, although patients with low-volume metastases will be eligible for prostate external beam radiotherapy:13

Finally, TERPs (NCT05223803) is a phase II trial of patients with de novo oligometastatic disease (<3 on conventional imaging or <5 on PET/CT) who will be randomized 1:1 to best systemic therapy + primary prostate radiotherapy +/- MDT (SBRT). The primary study endpoint is two-year failure-free survival.14
Conclusions and Future Directions
MDT has emerged as a guideline-recommended treatment option for prostate cancer patients with oligometastatic disease. The evidence to date suggests that MDT can be used in lieu of systemic therapy to delay time-to-hormone therapy or in combination with systemic therapy as a consolidative measure. The latest NCCN guidelines recommend considering SBRT for MDT in oligometastatic patients when ablation is the goal or in oligometastatic patients with limited progression or limited residual disease on otherwise effective systemic therapy (i.e., consolidation), where progression free survival is the goal.
The next ‘frontier’ of clinical trials for MDT will be evaluating its efficacy and safety in patients with PSMA-PET/CT-defined oligometastases and defining its role in the de novo metastatic hormone-sensitive setting. The results of these exciting trials will become available over the next few years.
Published August 2024
- Written by: Rashid K. Sayyid, MD MSc, University of Southern California, Los Angeles, CA & Zachary Klaassen, MD MSc, Wellstar MCG Health, Augusta, GA
- References:
- Foster CC, Pitroda SP, Weichselbaum RR. Definition, Biology, and History of Oligometastatic and Oligoprogressive Disease. Cancer J. 2020;26(2):96-9.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:6_suppl.
- Philips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-9.
- Deek MP, van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022;40(29):3377-82.
- Harrow S, Palma DA, Olson R, et al. Stereotactic Radiation for the Comprehensive Treatment of Oligometastases (SABR-COMET): Extended Long-Term Outcomes. Int J Radiat Oncol Bio Phys. 2022;114(4):611-6.
- Tang C, Sherry AD, Haymaker C, et al. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer: The EXTEND Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023;9(6): 825-34.
- Janssen J, Staal FHE, Brouwer CL, et al. Androgen Deprivation therapy for Oligo-recurrent Prostate cancer in addition to radioTherapy (ADOPT): study protocol for a randomised phase III trial. BMC Cancer. 2022;22(1):482.
- Roge M, Pointreau Y, Sargos P, et al. Randomized phase II trial in prostate cancer with hormone-sensitive OligometaSTatic relapse: Combining stereotactic ablative radiotherapy and durvalumab (POSTCARD GETUG P13): Study protocol. Clin Transl Radiat Oncol. 2023;40:100613.
- Nikitas J, Rettig M, Shen J, et al. Systemic and tumor-directed therapy for oligorecurrent metastatic prostate cancer (SATURN): Primary endpoint results of a phase II clinical trial. J Clin Oncol. 2024;42:Number 4_suppl.
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208-16.
- Glicksman RM, Metser U, Vines D, et al. Curative-intent Metastasis-directed Therapies for Molecularly-defined Oligorecurrent Prostate Cancer: A Prospective Phase II Trial Testing the Oligometastasis Hypothesis. Eur Urol. 2021;80(3):374–82.
- Nickols NG, Tsai S, Kane N, et al. Systemic and tumor-directed therapy for oligometastatic prostate cancer (SOLAR): A phase II trial for veterans with de novo oligometastatic prostate cancer. J Clin Oncol. 2024;42:Number 4_suppl.
- Connor MJ, Shah TT, Smigielska K, et al. Additional Treatments to the Local tumour for metastatic prostate cancer-Assessment of Novel Treatment Algorithms (IP2-ATLANTA): protocol for a multicentre, phase II randomised controlled trial. BMJ Open. 2021;11(2):e042953.
- Rana ZH, Helie N, Eggleston C, et al. Phase 2 randomized total eradication of metastatic lesions following definitive radiation to the prostate in de novo oligometastatic prostate cancer (TERPs) trial. J Clin Oncol. 2023;41:6_suppl.
American Society of Clinical Oncology (ASCO) 2024 Bladder Cancer Updates
EV+P nearly doubled median PFS and OS versus platinum-based chemotherapy in patients with previously untreated locally advanced or metastatic urothelial cancer in the phase 3 EV-302 trial and is NCCN category 1 and ESMO guidelines preferred treatment option. PRO assessments included the


