In May 2023, a novel tracer, flotufolastat F 18 (POSLUMA®), formerly referred to as 18F-rhPSMA-7.3, was approved by the FDA as a PSMA-PET tracer for the staging of prostate cancer patients with a suspected recurrence based on elevated PSA levels.3
While PSMA PET imaging offers improved diagnostic performance compared to conventional imaging, PET imaging using these radiotracers demonstrates low sensitivity in the biochemically recurrent setting, which may be secondary to low tumor uptake and retention or the short half-life of these radioisotopes.
Another important limitation of these radiotracers is that PSMA tumor expression is either absent or low in up to 25% of men with biochemical recurrence or castrate-resistant disease, 4-6 which has important diagnostic and therapeutic implications. Accordingly, there is a need to identify and evaluate novel prostate cancer-specific cell surface targets.
In this Center of Excellence article, we will discuss new PET radiotracers and targets for the staging of biochemically recurrent prostate cancer patients.
Flotufolastat F 18 (POSLUMA®)
Flotufolastat F 18 is an optimized, high-affinity radiohybrid (rh) PSMA-targeted PET imaging agent that can be efficiently labeled with 18F or with radioactive metal isotopes and, consequently offers both diagnostic and therapeutic PSMA targeting.7
Flotufolastat F 18 demonstrates high PSMA binding affinity, high internalization by PSMA-expressing cells, medium-to-low lipophilicity, and high human serum albumin binding, which together can provide optimal kidney clearance.8-10 Flotufolastat F 18 may have lower average urinary excretion compared to 68Ga PSMA-11 and 18F-DCFPyL,7 which may facilitate improved imaging in the pelvic region and may be useful for treatment planning in the salvage radiotherapy setting.
This radiotracer was FDA approved in the biochemically recurrent setting based on the results of the phase III SPOTLIGHT trial.
SPOTLIGHTSPOTLIGHT (NCT04186845) was a multicenter, open-label, single-arm phase III trial of men with suspected biochemical recurrence following radical prostatectomy, radiotherapy, brachytherapy, or focal gland therapy.11 Patients were required to have discontinued any ADT ≥16 weeks prior to screening. All patients underwent flotufolastat F 18 PSMA PET/CT plus standard of care imaging (bone scan, abdominopelvic CT or MRI, chest CT, 18F-NaF-PET, or 18F-fluciclovine-PET) ≤2 weeks post-flotufolastat F 18 PET/CT.
For flotufolastat F 18 PET/CT-positive patients, the images were initially interpreted by trained local readers. Where safe and medically feasible, biopsies of metastatic lesions were recommended as ‘standard of truth’. If not feasible or the patient declined, confirmatory imaging (functional or multiparametric MRI, CT, 18F-fluciclovine-PET or 18F-NaF-PET as per site standard of care) was recommended ≤90 days post-PET to confirm flotufolastat F 18 PSMA PET/CT findings. For the efficacy analysis, all PET images were interpreted by three blinded independent central readers who had received image interpretation training.
SPOTLIGHT had two co-primary endpoints, which are both newly FDA-required endpoints that were not previously used in phase 3 registration trials for PSMA-PET agents:
- Verified detection rate = Detection rate x positive predictive value
- Combined region-level positive predictive value:
- Incorporates every PET-positive region (≤3) from all PET-positive patients into the positive predictive value formula. This endpoint relies heavily on imaging as standard of truth since it is usually neither ethical nor feasible to obtain multiple biopsies in the same patient.
A scan was considered a true positive if it had ≥1 standard of truth-confirmed PET-positive region. A region was considered true positive if ≥1 PET-positive lesion in that region was confirmed by standard of truth, irrespective of any coexisting false positive lesions. Statistical thresholds (lower bounds of the confidence intervals) of 36.5% and 62.5% were prespecified for verified detection rate and combined region-level positive predictive value, respectively. A key secondary endpoint was the positive predictive value (i.e., correct localization rate), which is the traditional metric used in CONDOR and the 68Ga-PSMA-11 registration trial.12,13 This definition allows for a patient to be considered a true positive by standard-of-truth, irrespective of other PET-positive lesions that were categorized as false positive.
The study cohort included 366 patients with data available to determine standard-of-truth (histopathology: 69; imaging: 297). The median time from the initial prostate cancer diagnosis was 70 months. Overall, 77% of patients had undergone a prior radical prostatectomy, 50% of whom had received additional adjuvant/salvage radiotherapy. The median baseline PSA was 1.27 ng/ml.
The overall detection rate among all patients with an evaluable flotufolastat F 18 PSMA PET/CT scan was 83% by majority read. The detection rate at a PSA <0.5 ng/ml was 63%, higher than that observed with 68Ga-PSMA-11 (38%) and 18F-DCFPyL PET/CTs (36%).12,13 The overall inter- and intra-reader agreements were >75% and >85%, respectively:
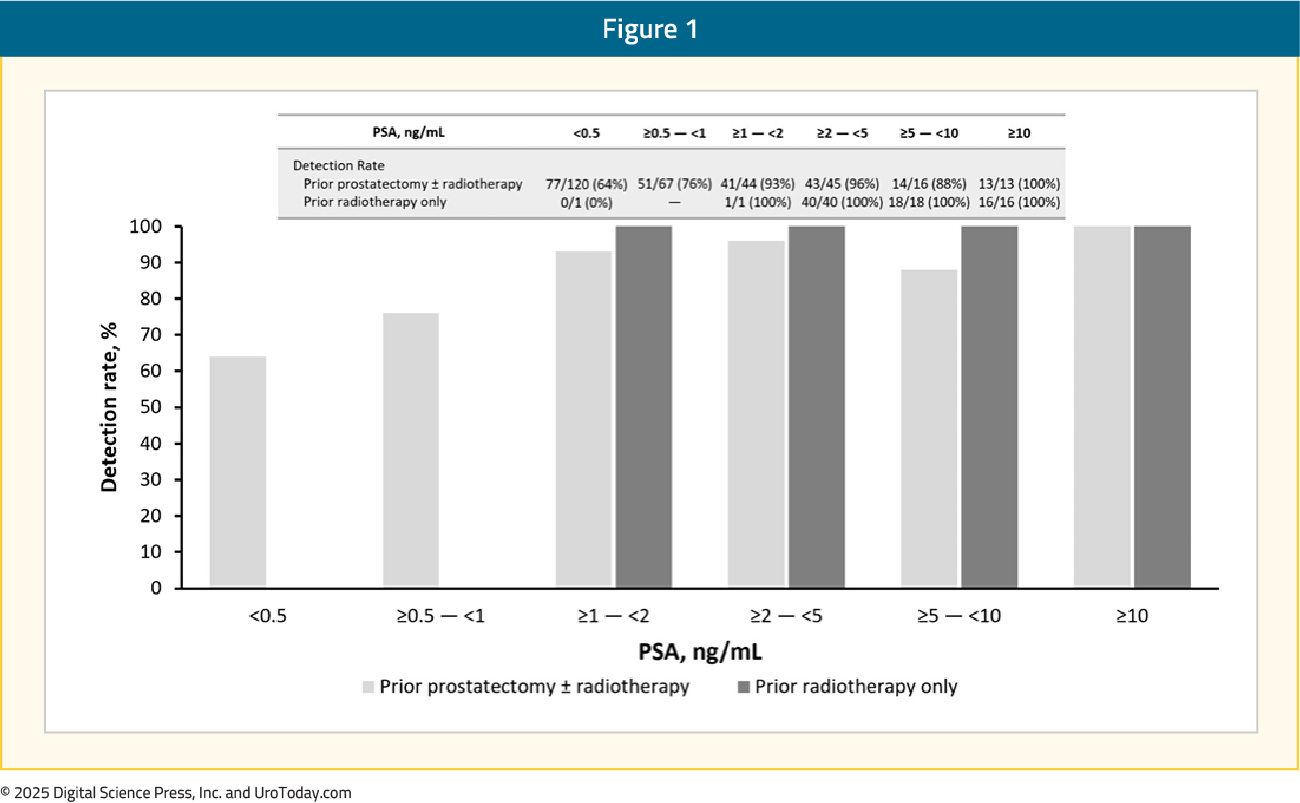
The verified detection rate ranged from 51% (95% CI: 46.1–56.6) to 54% (95% CI: 48.8–59.3), exceeding the prespecified statistical threshold. The prespecified statistical threshold was exceeded for all three readers (p < 0.0001 for each), thus meeting the co-primary verified detection rate endpoint. On a regional level, the verified detection rate by majority read was as follows:
- Prostate/prostate bed: 16%
- Pelvic lymph nodes: 22%
- Extra-pelvic sites: 31%
The verified detection rate improved with increasing baseline PSA, with the majority read verified detection rate ranging from 31% at PSA <0.5 ng/mL to 84% at PSA ≥10 ng/mL:
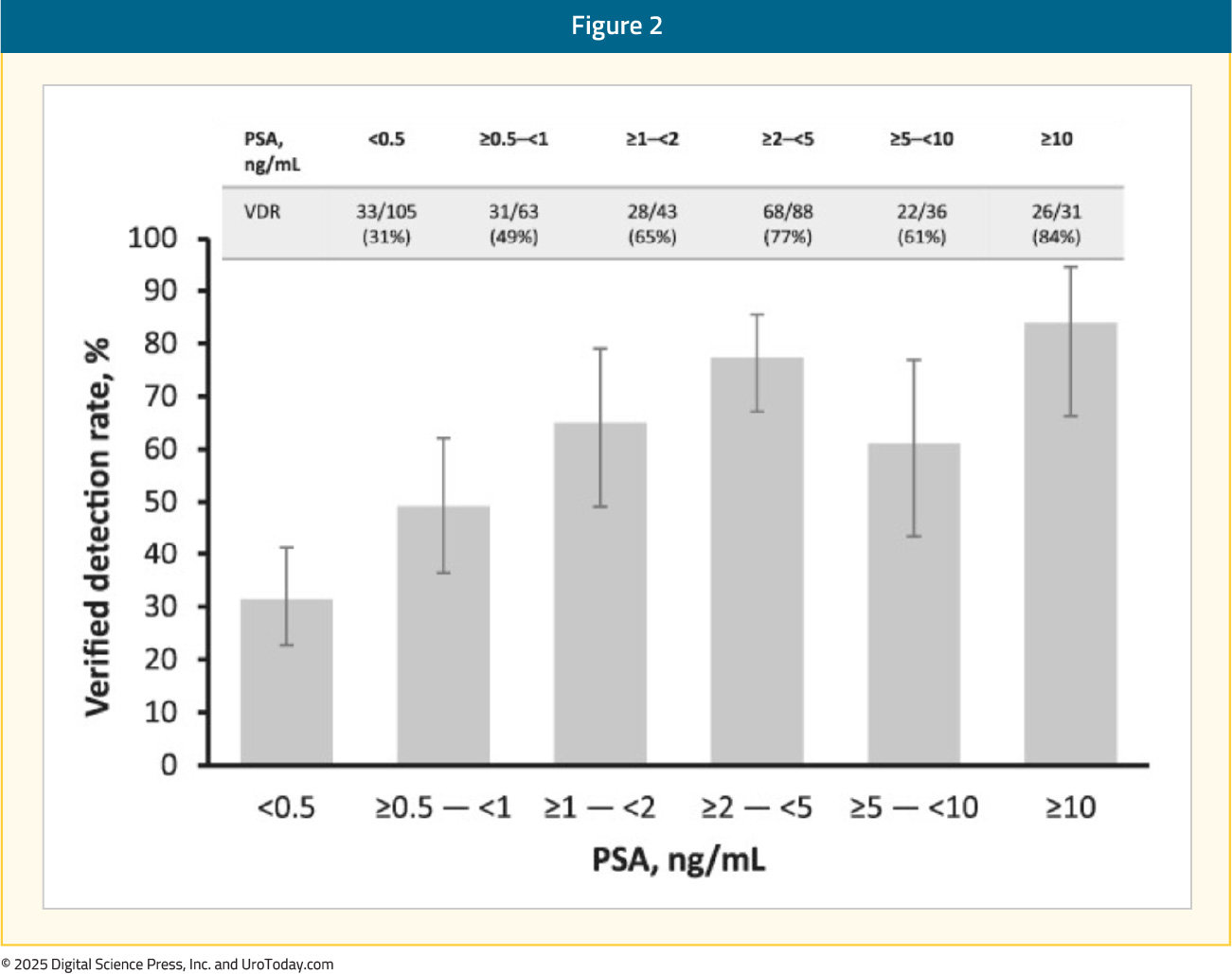
The second co-primary endpoint, combined region-level positive predictive value, ranged between 46% and 60% across the readers (majority read: 60%), with the lower bound of the 95% CI not exceeding the prespecified threshold for this endpoint. In the subgroup with histopathology standard-of-truth, the majority read combined region-level positive predictive value was 72%:
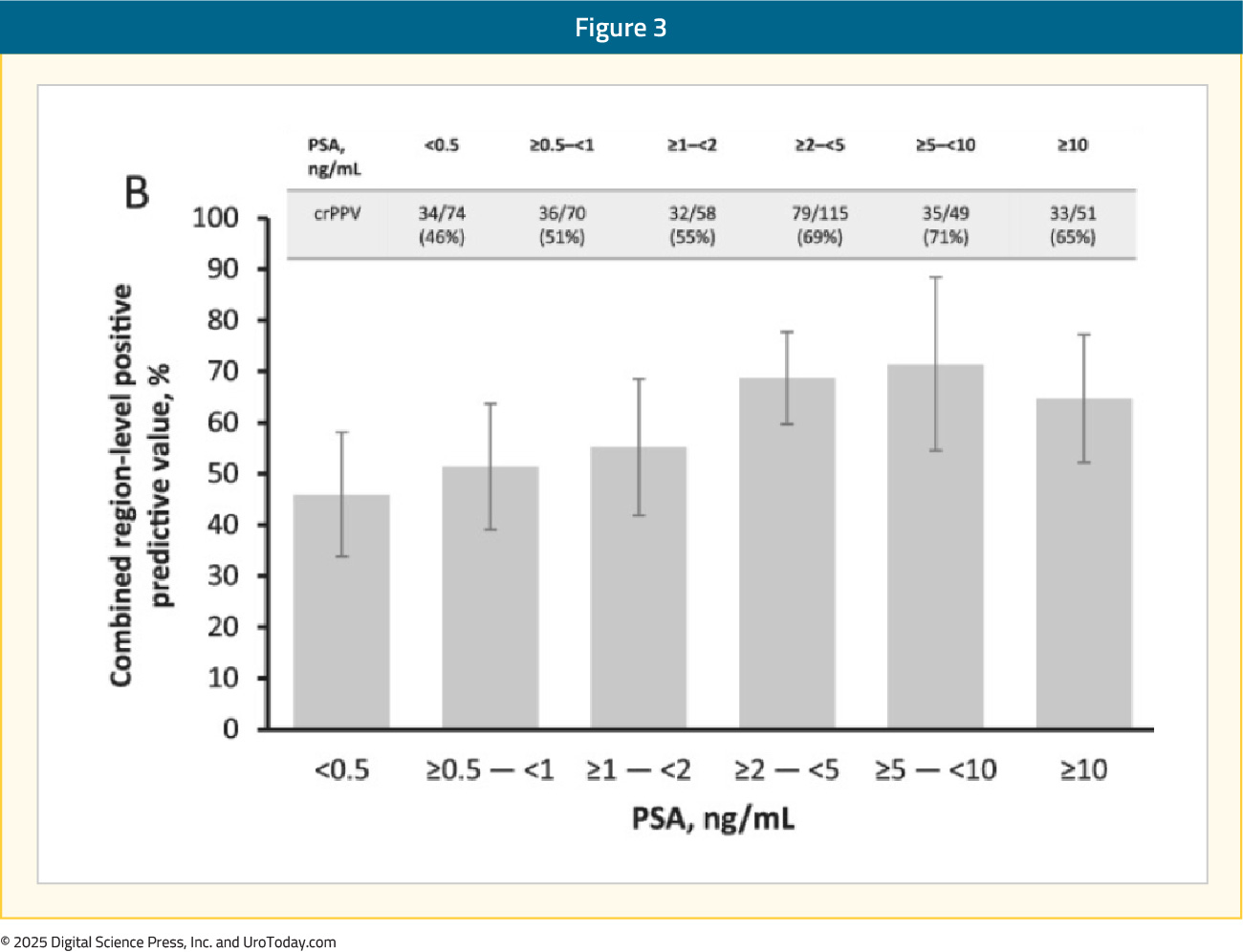
The overall patient-level positive predictive value (i.e., correct localization rate) was 65% on the majority read. In those with histopathology standard-of-truth available, the positive predictive value was higher at 82%, highlighting the limitations of using conventional imaging to confirm PET/CT imaging findings. No serious radiotracer-related adverse events were reported.11
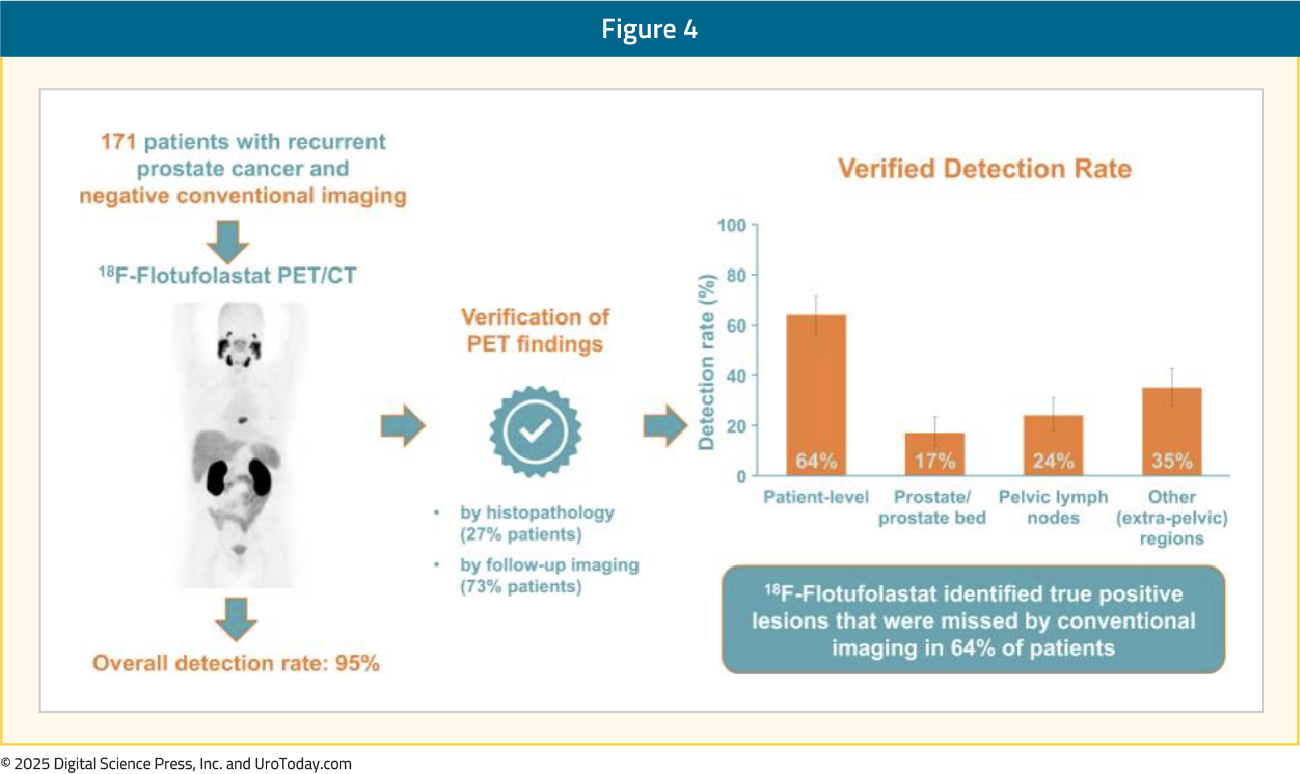
Flotufolastat F 18 PET/CT in Biochemically Recurrent Patients with Negative Conventional ImagingA follow-up analysis of biochemically recurrent patients with negative baseline conventional imaging from SPOTLIGHT was recently published in The Journal of Nuclear Medicine.14 In total, 171 patients with negative baseline conventional imaging and standard-of-truth verification by histopathology or post-PET confirmatory imaging were included. By majority read, the overall detection rate among these patients was 95%, and 64% had ≥1 true positive lesion identified (i.e., verified detection rate). Among the radical prostatectomy recurrence cohort, 8.3% of patients had ≥1 true-positive lesion in the prostate bed, 28% in pelvic lymph nodes, and 35% in other sites. Among those who had received radiotherapy, 50% had true-positive detections in the prostate, 8.3% in pelvic lymph nodes, and 36% in other sites:
64Cu-SAR-bisPSMA
64Cu-SAR-bisPSMA is a next generation radiopharmaceutical developed by Clarity Pharmaceuticals. It utilises copper isotopes for imaging (64Cu) and therapy (67Cu). The targeting moiety, linked to a SAR chelator, has two PSMA-targeting functional groups, which exhibit increased tumor uptake and retention.
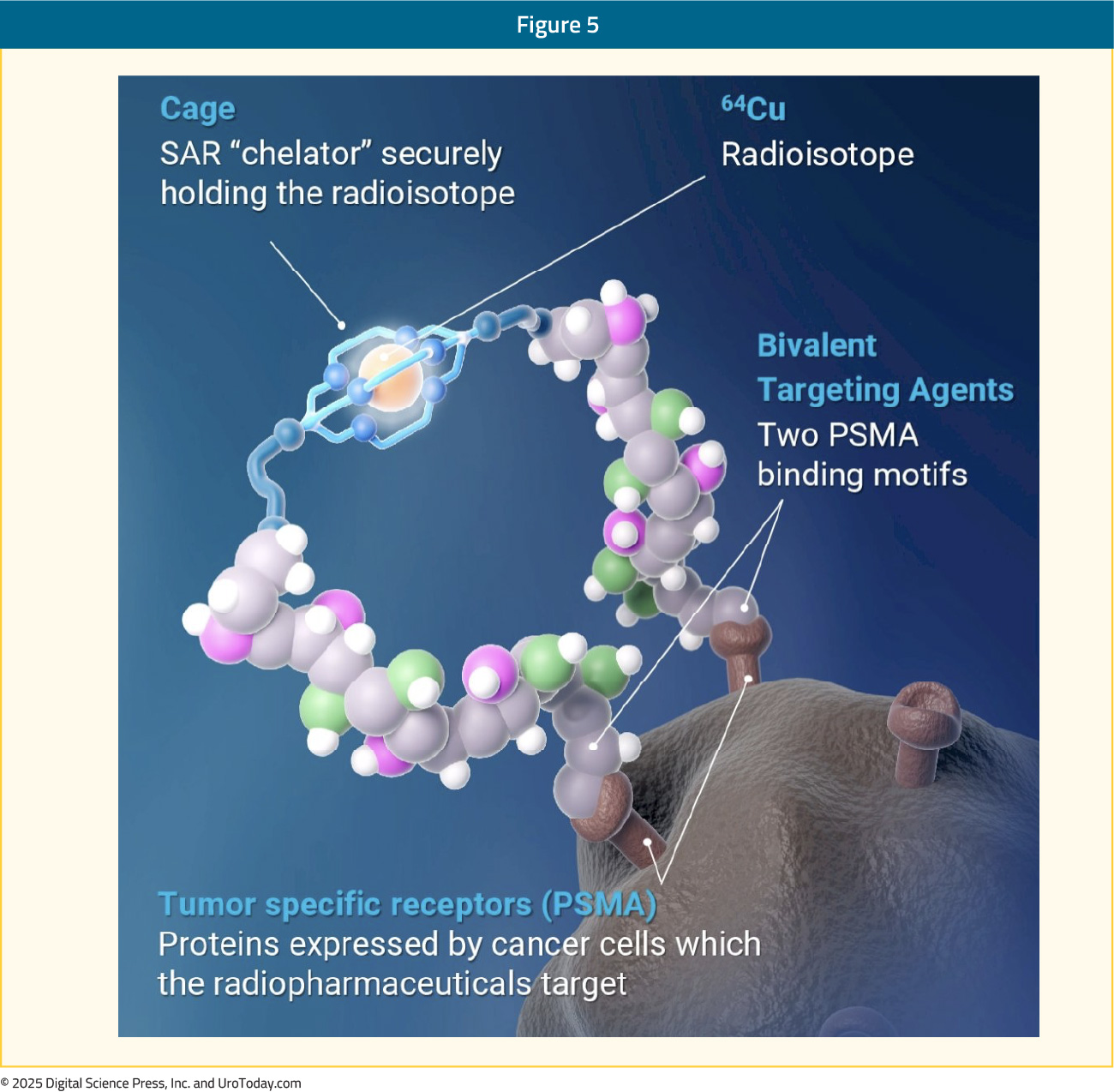
64Cu has a long half-life of 12.7 hours, compared to 1.1 and 1.83 hours for 68Ga and 18F radiotracers, respectively. This allows for a much longer product shelf life (up to 48 hours versus 4 and 10 hours for 68Ga and 18F, respectively) and imaging windows (1–30 hours versus 50–100 and 60–90 mins for 68Ga and 18F, respectively). Additionally, 64Cu has a shorter positron range (0.56 mm), leading to improved scan resolution.
COBRA: Phase I/II Trial in Biochemical Recurrence SettingCOBRA was a phase I/II study assessing the safety and efficacy of 64Cu-SAR-bisPSMA (200 MBq) in prostate cancer patients with biochemical recurrence and negative or equivocal standard of care imaging. Patients underwent PET/CT on Day 0 and Day 1 (1–4 hours and 24 ± 6 hours post-dose, respectively), interpreted by three blinded central readers. The reference standard was either histopathology, standard of care imaging, or PSA response, as determined by an independent, blinded, central expert panel. The study design is as follows: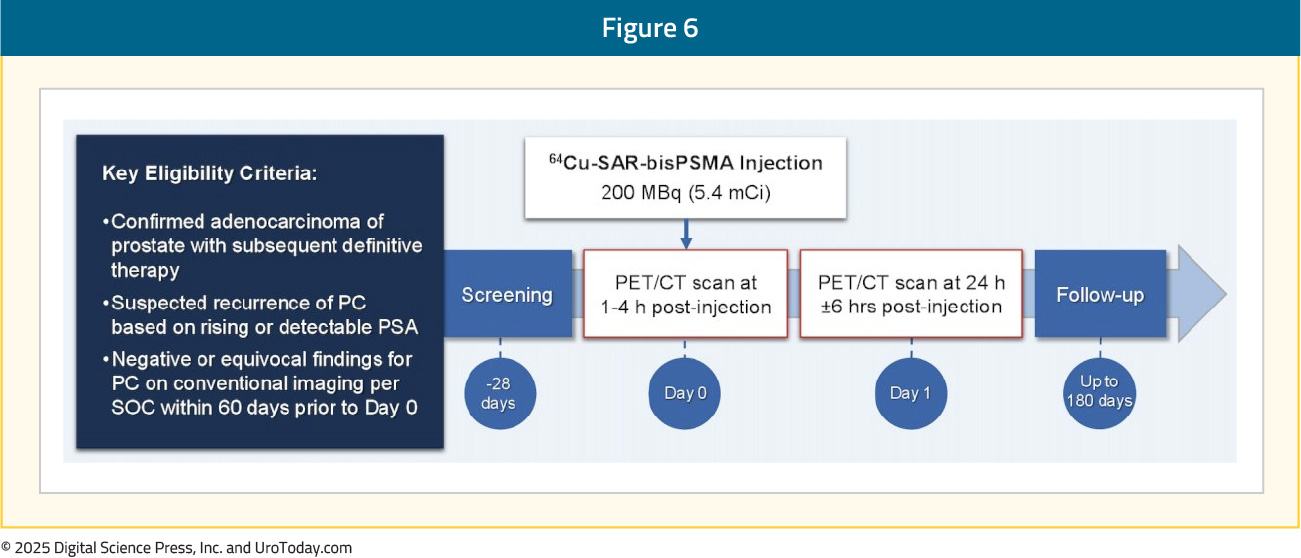
The efficacy cohort included 42 patients with an available reference standard. There was one tracer-related adverse event recorded – grade 2 worsening of type II diabetes that subsequently resolved. The total number of lesions identified, across all three readers, increased between Days 0 and 1 from 70 to 129, representing an 82% absolute increase in the number of lesions detected. The Day 0 detection rate across the readers was 44–58%, increasing on Day 1 to 58–80%, representing a 34% increase in patients having a positive 64Cu-SAR-bisPSMA scan on Day 1 (71%) versus Day 0 (53%) across the average of three readers:
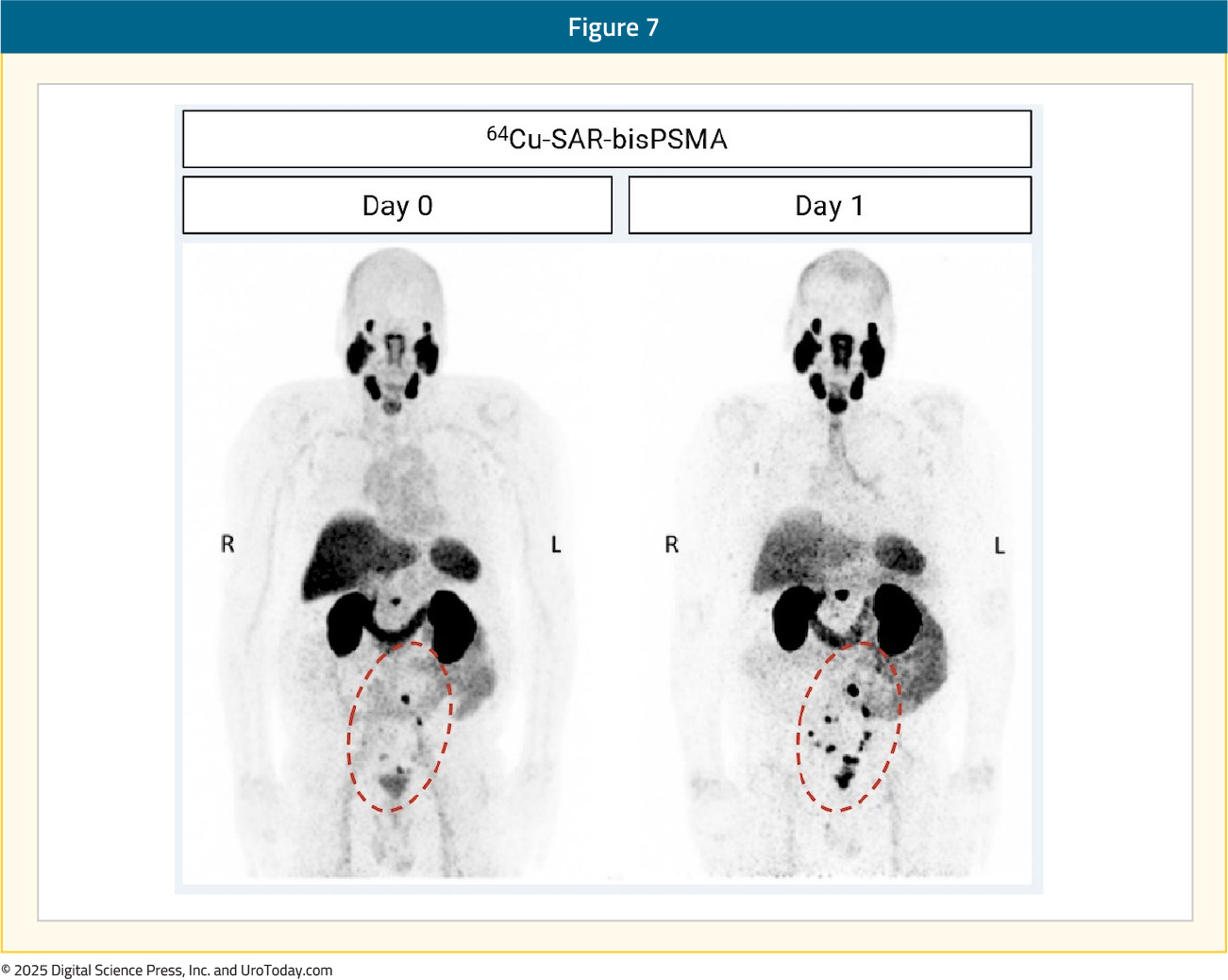
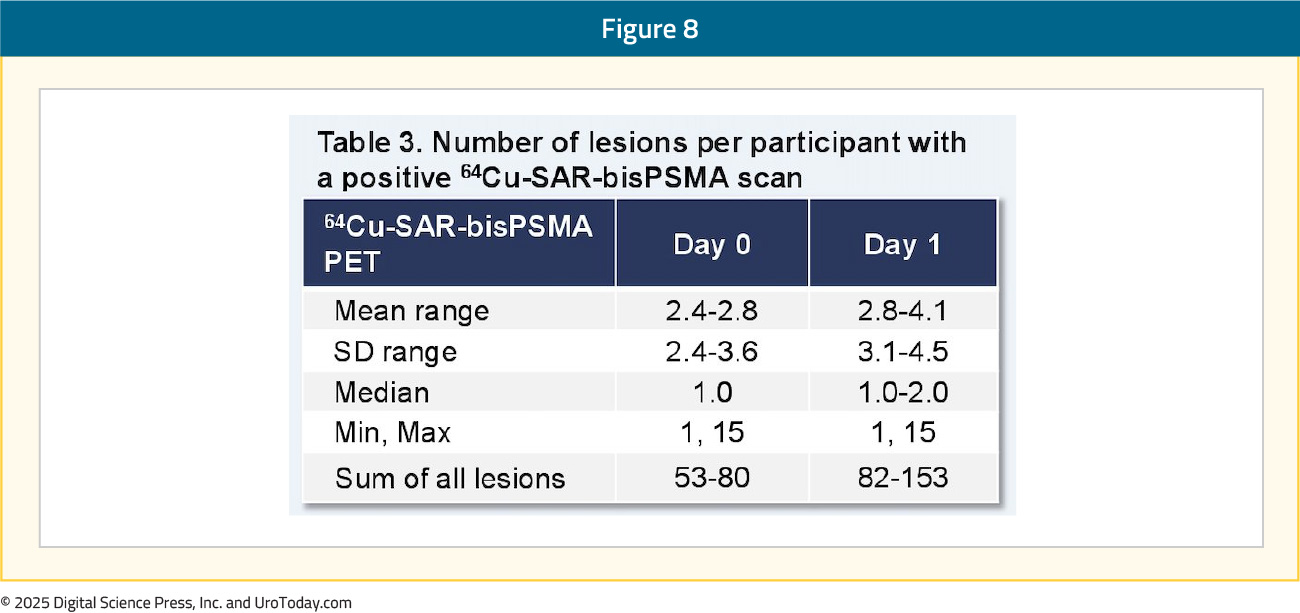
Pelvic lymph nodes had a Day 0 positive predictive value range of 71–88% versus 50–62% on Day 1. The relative decrease in the Day 1 positive predictive value was related to challenges in obtaining the reference standard for additional lesions identified on Day 1, where biopsy of all lesions was not feasible, and due to the low sensitivity of current standard of care imaging used for co-localization:
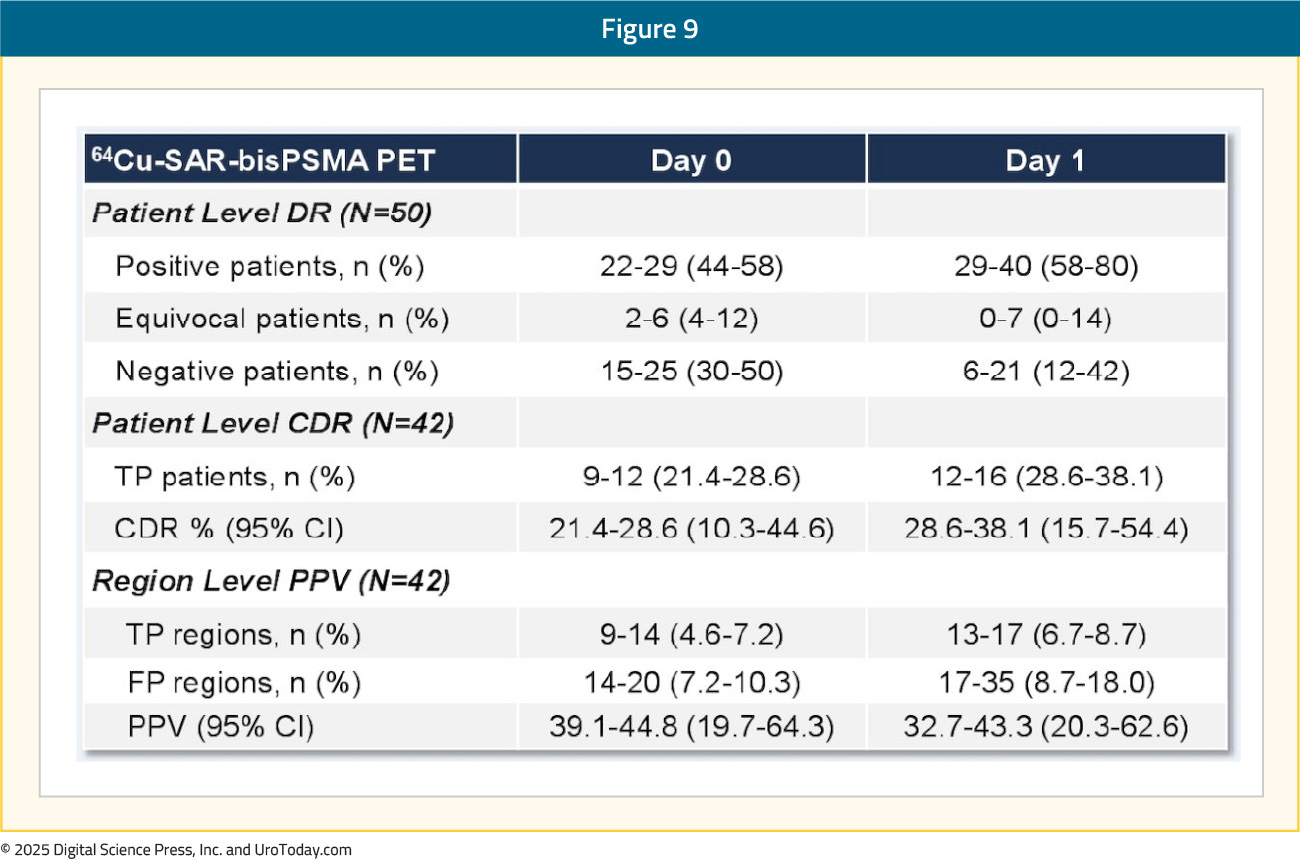
The use of 64Cu-SAR-bisPSMA PET imaging was associated with management plan changes in 48% of patients. Notably, 64Cu-SAR-bisPSMA PET/CT was able to detect lesions in the 2 mm range, with currently approved radiotracers limited in their ability to detect micrometastases <5 mm in size:
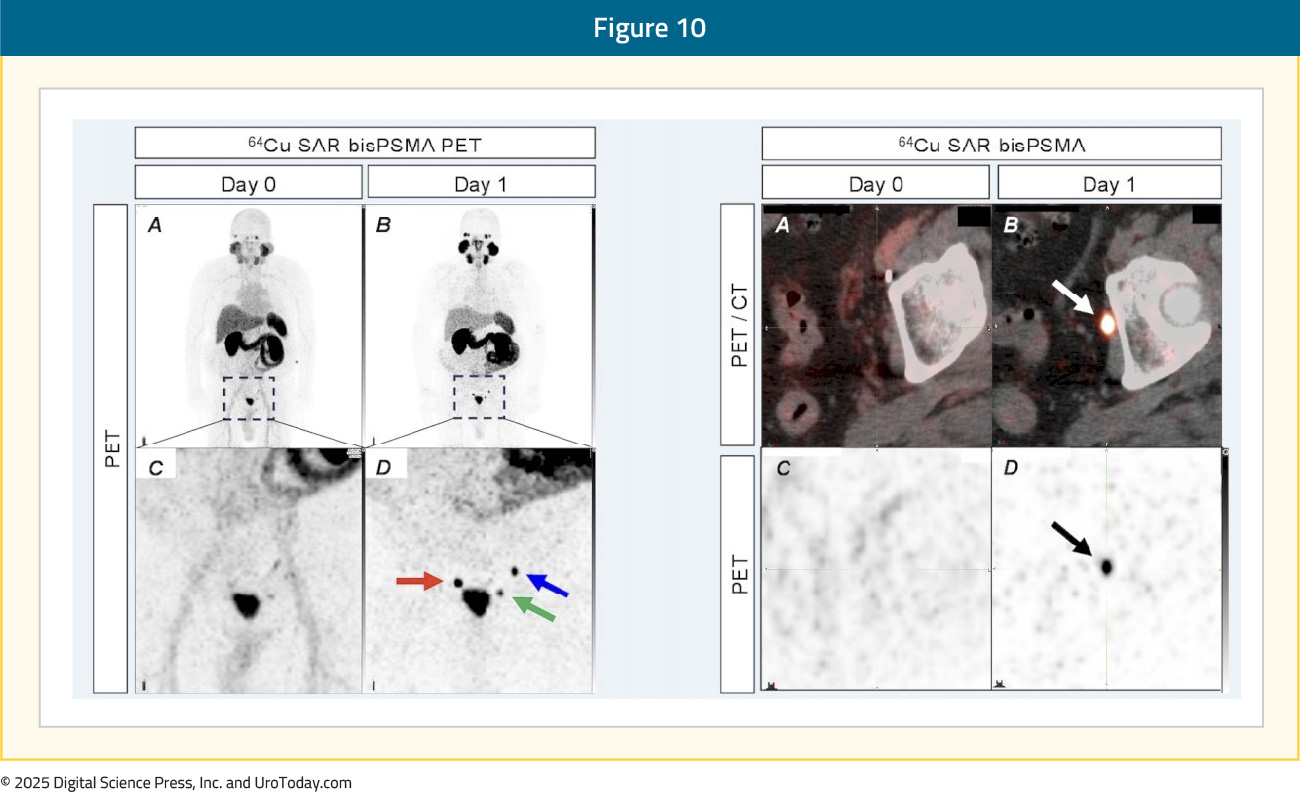
The results of the COBRA trial demonstrated that 64Cu-SAR-bisPSMA PET is safe and effective for detecting prostate cancer lesions in biochemically recurrent prostate cancer patients with negative or equivocal conventional imaging. 64Cu-SAR-bisPSMA PET was able to detect lesions, as small as 2 mm, in up to 80% of patients. Notably, next-day imaging, as compared to same-day imaging, was able to detect a greater number of lesions in more patients, a feature unique to 64Cu-SAR-bisPSMA PET imaging.15
Gastrin-releasing Peptide Receptor-targeted Agents
GRPr is a cell membrane receptor overexpressed in various cancers, including early and advanced stage prostate cancer. GRPr is a G protein-coupled receptor from the bombesin (BBN) family that is involved in cell growth, proliferation, angiogenesis, and migration. The most well-recognized ligand of GRPr is bombesin, which in its bound form activates the PI3K/AKT and the MAPK signaling pathways.16 The clinical significance of GRPr expression in prostate cancer cells remains controversial, with conflicting data regarding the association of GRPr expression with tumor grade:17,18
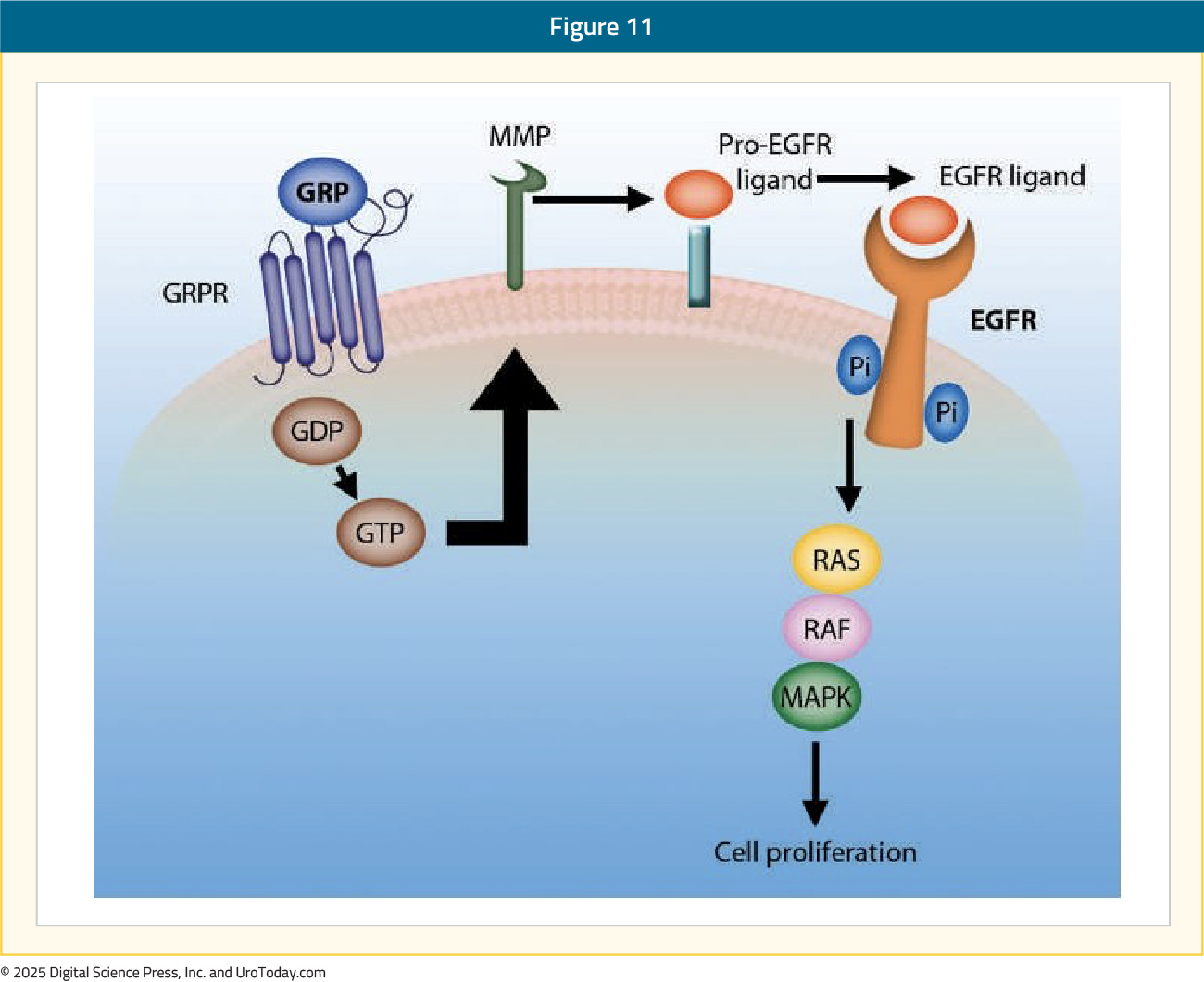
RM2 is an anti-GRPr antibody that can be labeled with either 68Ga or 177Lu for imaging and theranostic purposes, respectively. 68Ga-DOTA-RM2 PET/MRI has demonstrated encouraging diagnostic performance characteristics in patients with evidence of biochemically recurrent prostate cancer. Among 35 patients with biochemically recurrent prostate cancer, with a mean PSA of 1.88 ng/ml, all patients underwent a 68Ga PSMA PET/MRI for restaging purposes, with 31/35 also undergoing 68Ga-DOTA-RM2 PET/MRI scan within 16 days. A higher detection rate was observed for 68Ga-PSMA PET/MRI, with more lesions being detected compared to 68Ga-DOTA-RM2 PET/MRI (26/35 versus 15/31 patients, p = 0.016; 95 versus 41 lesions, p = 0.002). There were discordant lesions in 11/31 patients, 10 of which were 68Ga-PSMA positive (positive predictive value = 90%). PSMA PET imaging demonstrated higher detection for higher-grade tumors, while GRPr PET imaging had higher detection for lower-grade tumors.19
In the mCRPC setting, a retrospective comparison of 68Ga-RM2 and 68Ga-PSMA-PET/CT was performed in 57 patients to identify RM2 or PSMA positivity as a prerequisite for radionuclide treatment with either 177Lu-RM2 or 177Lu-PSMA. Among these patients, three did not have evidence of PSMA- or RM2-positive lesions on visual assessment. The remaining 54 patients had evidence of PSMA uptake; among 54 PSMA-positive patients, 37 had evidence of RM2 uptake (9 higher, 28 lower). There were discordant PET/CT findings (i.e., PSMA+/RM2- or PSMA-/RM2+) in 4/57 patients. Overall, SUVmax and SUVpeak were significantly higher for PSMA-PET/CT, compared to RM2-PET/CT (p < 0.001). When stratified by recurrent disease site, there was evidence of more intense uptake, as quantified by SUVmax and SUVpeak, in locally recurrent (p = 0.051), nodal (p < 0.001), skeletal (p < 0.001), and hepatic lesions (p = 0.026).20
64Cu-SAR-BBNThere are numerous ongoing trials evaluating the GRPr-targeted agent, 64Cu-SAR-BBN. The phase II BOP trial was designed to assess the safety and diagnostic performance of 64Cu-SAR-BBN in two prostate cancer cohorts: (i) biochemically recurrent patients with negative PSMA PET imaging scans or low PSMA expression and (ii) mCRPC patients not suitable for PSMA therapy. Study results of the biochemically recurrent cohort were presented at the 2023 European Association of Nuclear Medicine conference. The mean PSA at study entry was 0.69 ng/ml, with 96% of patients having undergone a radical prostatectomy. Participants in the BOP trial received the mean dose of 210 MBq of 64Cu SAR-BBN and underwent PET imaging at 1-, 3- and 24-hours post-radiotracer administration. No adverse events from 64Cu-SAR-BBN administration were reported. 64Cu SAR-BBN PET-avid disease was identified in over 30% of patients with negative or equivocal standard of care PSMA PET (8/25, 32% detection rate).
SABRE is an ongoing phase II, single arm, non-randomized, open-label study of 64Cu-SAR-BBN in biochemically recurrent patients with negative or equivocal findings on a PSMA PET (n = 50). The study objectives are to assess the safety of 64Cu-SAR-BBN (200 MBq), with efficacy endpoints including the correct detection rate (participant level) and positive predictive value (at participant and region level). Patients will complete a PET/CT on the same day (1 to 4 hours) and the next day (24 ± 6 hours) post-dose. The 64Cu-SAR-BBN PET/CT scans will be assessed centrally by three independent, blinded readers. The 64Cu-SAR-BBN PET/CT results will then be assessed against a composite reference standard (histopathology, conventional imaging, and/or PSA levels) determined by an independent, blinded, central expert panel.21
Conclusions
There are numerous radiotracers being developed and evaluated for the staging of biochemically recurrent prostate cancer. These radiotracers offer both diagnostic and theranostic potential, which is of particular significance in advanced treatment settings. Furthermore, novel radiotracers that bind to non-PSMA cell surface targets may overcome the limitations of low or absent PSMA expressing-tumors in advanced disease states. These radiotracers may add to the arsenal of PET imaging tools available to accurately localize the sites of disease recurrence in the biochemically recurrent setting and allow for personalized, targeted treatment approaches, irrespective of tumor PSMA expression level.
Published January 2025


