Introduction
Intravesical Bacillus Calmette-Guerin (BCG) currently remains the standard-of-care, guideline recommended treatment of choice in the adjuvant setting for intermediate- and high-risk non-muscle invasive bladder cancer (NMIBC) due to its ability to reduce the risk of disease recurrence and, more importantly, disease progression.1-3 However, despite adequate BCG treatment, defined as receipt of at least five doses of the initial six-dose induction course and at least 2/3 maintenance doses or at least 2/6 doses of the second induction course, up to 50% of such patients will develop a BCG-refractory, relapsing, or failure state.4 Currently, radical cystectomy remains the gold standard approach in this setting.1 However, many patients are either unfit or refuse cystectomy. As such, bladder-sparing approaches in this setting are of utmost importance.
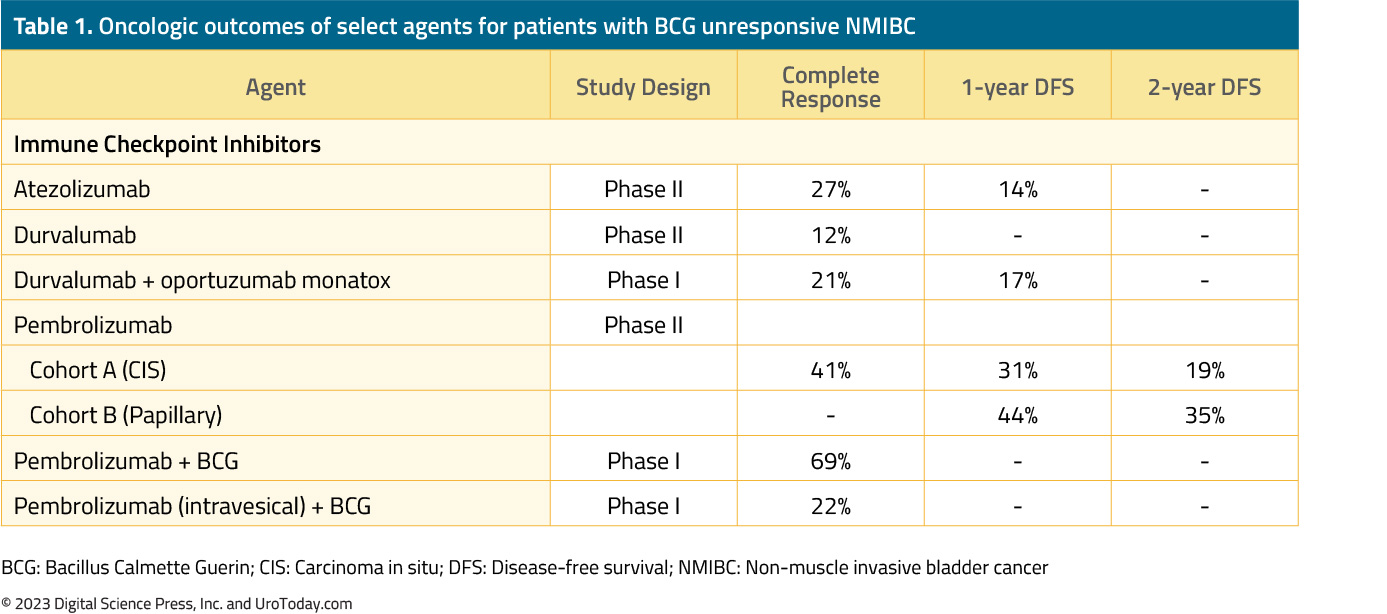
In 1998, the United States Food and Drug Administration (FDA) approved Valrubicin (Valstar) for the intravesical therapy of patients with BCG-refractory carcinoma in situ (CIS) for whom cystectomy would be associated with unacceptable morbidity or mortality. However, the efficacy of Valstar in this setting was limited with only 21% of patients having a complete response, with a median response of only one year.5 In an effort to address this shortage of options, in 2018 the FDA issued a guidance document to aid in the development of drugs and biologics in this disease space. In an attempt to standardize disease definitions and facilitate clinical trial design, BCG-unresponsive NMIBC was defined as follows:6
- Persistent or recurrent CIS within 12 months of adequate BCG therapy
- Recurrent high-grade Ta/T1 disease within six months of completion of adequate BCG therapy
- High-grade T1 disease detected on the first evaluation following an induction BCG course
One of the etiologies of BCG failure may be the adaptive immune response, with non-responders having higher baseline Programmed Cell Death Ligand 1 (PD-L1) levels (25-28% compared to 0-4% in responders), which may lead to increased co-localization with CD8+ T cells.9 As such, it has been hypothesized that anti-programmed cell death protein 1 (PD-1)/PD-L1 inhibitors, alone or in combination with other agents, may have potential benefit in this disease space. In this Center of Excellence article, we will discuss the currently available evidence and key ongoing studies for immune checkpoint inhibitor therapy in the BCG-unresponsive NMIBC disease space.
Pembrolizumab
KEYNOTE-057: Cohort A
The KEYNOTE-057 trial is an open-label, single-arm, multicenter, phase II trial consisting of two adult patient cohorts:
- Cohort A: BCG-unresponsive CIS +/- papillary tumors
- Cohort B: BCG-unresponsive high-grade Ta or any grade T1 papillary tumors (no CIS)
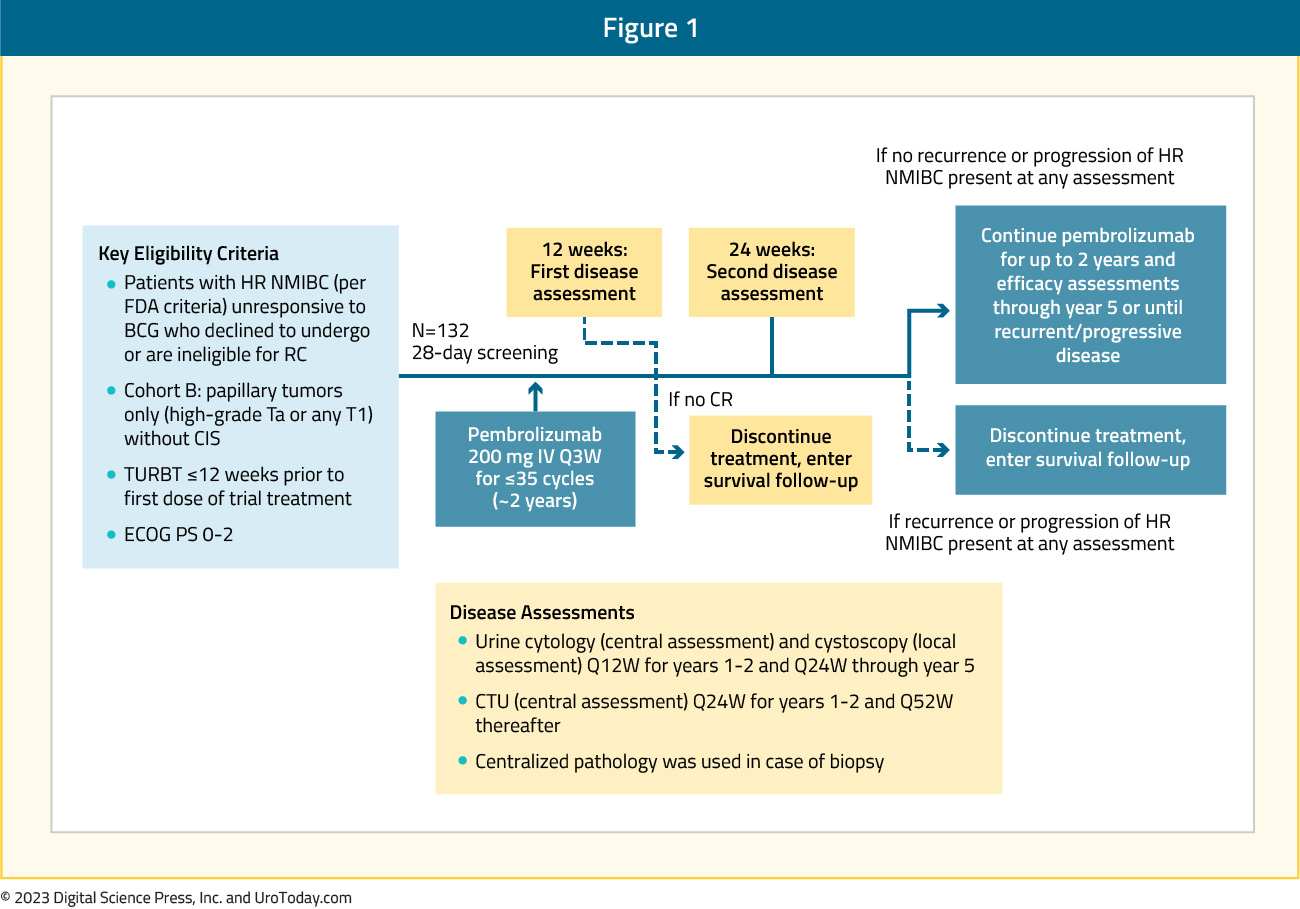
Patients in this trial received intravenous pembrolizumab (KEYTRUDA®), an anti-PD-1 agent, at 200 mg every 3 weeks for up to 24 months or until patients had evidence of centrally-confirmed disease persistence, recurrence, or progression or unacceptable drug-related toxicity. Patients underwent a cystoscopy and had urine cytology performed every three months for the first two years and every six months thereafter for up to five years. There were no protocol-mandated biopsies, which were only performed in case of an abnormal cytology or cystoscopic findings. The 36-months follow-up outcomes of the 101 patients in Cohort A were published in The Lancet Oncology in 2021.10 Overall, 64% of patients had CIS-only disease, and the 3-month complete response rate, defined as absence of high-risk NMIBC or progressive disease, was 41%.

Among those who achieved a complete response, 53% maintained a complete response for >9 months. Grade 3-4 treatment-related adverse events were observed in 13% of patients, the most common of which were arthralgia (2%), malaise (1%), and pruritis (1%).
KEYNOTE-057: Cohort B
Results of Cohort B (papillary-only disease) were recently presented at The American Society for Clinical Oncology (ASCO) Genitourinary (GU) Cancer Symposium 2023. This cohort included 132 patients, with a median follow-up of 45.4 months, with 43% of patients having T1 disease. The median age was 72 years and almost 80% were male. The median number of prior BCG installations was 10 (range: 6 to 33), and the median number of pembrolizumab cycles received was 9.5. The 12-month disease-free survival rate (primary outcome) was 43.5% (95% CI: 35 to 52%), and the corresponding 24- and 36-months rates were 35% for both. Freedom from disease progression, defined as grade or stage worsening, development of invasive or metastatic disease or death, was observed in 88.2% of patients. The one-year overall survival rate was 96.2%. Grade 3-4 treatment-related adverse events were observed in 14.4% of patients, with 10.6% discontinuing therapy due to such events.11
Pembrolizumab (Intravenous) + BCG
The combination of intravenous pembrolizumab and intravesical BCG has recently been evaluated in a phase 1 dose-escalation trial of patients with high grade NMIBC who had persistent or recurrent disease following prior BCG. In this study, 13 patients received six doses of pembrolizumab every 3 weeks over 16 weeks, with concurrent six weekly doses of BCG beginning at week 7. There were nine grade 3 adverse events, the most common of which were cardiomyopathy and musculoskeletal (wrist pain, arthritis), and one grade 4 adverse event (adrenal insufficiency). Of the 13 patients treated, two died during the study follow-up. The 3-months complete response rate was 69%.12
Pembrolizumab (Intravesical) + BCG
The combination of intravesical pembrolizumab and BCG was tested in a 3 + 3 dose escalation, phase 1 trial in patients with BCG-unresponsive NMIBC. Of note, this study was terminated early due to the COVID-19 pandemic. Nine patients received intravesical pembrolizumab (1 – 5 mg/kg for 2 hours) starting two weeks prior to BCG induction and continued until recurrence. The median follow-up was 35 months, and 5/9 patients were still alive at the end of the trial. Pembrolizumab was detectable in the urine, but not blood samples of these patients. No maximum tolerated dose was reached; however, one dose-limiting toxicity, grade two diarrhea, was reported. There was one death that was deemed potentially related to study treatment (myasthenia gravis). The six- and 12-month recurrence-free rates were 67% and 22%, respectively.13 The trial design and outcomes of this trial are summarized as follows:

Durvalumab
Durvalumab
Durvalumab (IMFINZI®) is a human IgG1 monoclonal antibody that inhibits PD-L1. A recently reported phase II trial evaluated intravenous durvalumab 1,500 mg given every 4 weeks for up to 12 months in patients with BCG-unresponsive CIS. The primary endpoint of 6-months complete response was defined by a negative cystoscopy, urine cytology, and absence of high-grade recurrence on bladder mapping biopsy. Between March 2017 and January 2020, this trial accrued 17 patients, of whom four were withdrawn from the study due to evidence of persistent CIS on biopsy sampling. A complete response at 6 months was observed in only 12% of patients. The duration of response in the two responders was only 10 and 18 months. Immune-related adverse events were observed in 41% of patients, with a single Grade 3 lipase elevation attributed to durvalumab.14
Durvalumab + Vicinium
Vicinium (Oportuzumab Monatox, VB4-845) is a recombinant fusion protein comprising a humanized anti-EpCAM single-chain antibody linked to Pseudomonas exotoxin A. Once bound to the cancer cell, Vicinium is internalized, and the toxin moiety is released into the cytosol where it induces apoptosis. Vicinium monotherapy has been previously evaluated in patients with CIS who had failed prior BCG. This agent was associated with a 3-month complete response rate of 44%, with a median duration of response of 274 to 408 days.15
The combination of durvalumab and Vicinium has been more recently evaluated in a phase 1 trial of patients with high grade NMIBC previously treated with BCG. This phase 1 trial enrolled 12 patients with BCG unresponsive NMIBC (5 high grade Ta, 3 high grade T1, 4 CIS). All 12 patients had ≥1 treatment-related adverse event, but only 8% were ≥3. The most common adverse event observed was urinary frequency in 41% of patients. The 3- and 6-month complete response rates were 41% and 33%, respectively. Of the four patients who were disease free by the 6th month, two had no evidence of recurrence after >12 months of follow-up.16
Atezolizumab
SWOG S1605 is a single-arm, phase II trial testing systemic atezolizumab (TECENTRIQ®), an anti-PD-L1, in patients with BCG-unresponsive high-risk NMIBC who are either unfit for or declined radical cystectomy. Treatment consisted of intravenous atezolizumab 1,200 mg every 3 weeks for up to one year. The co-primary endpoints were pathological complete response rate at 6 months in patients with CIS, and event-free survival in all patients at 18 months. Among the 74 CIS patients in the efficacy cohort, a pathologic complete response at 6 months was attained in 20 patients (27%). Among these 20 patients, the 12-month recurrence-free rate was 49%, and the median duration of response was 15.4 months.
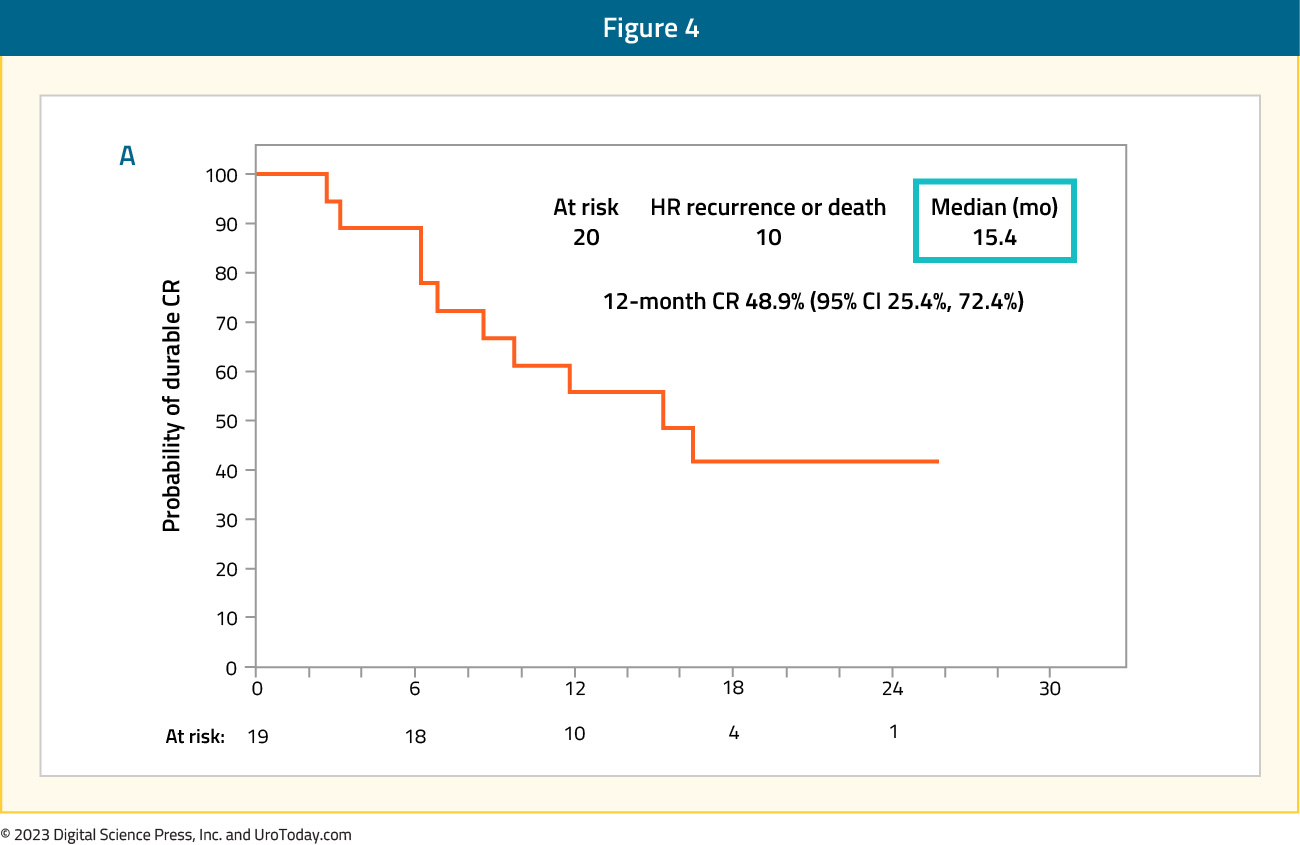
The overall event-free survival rate at 18 months was:
- Overall cohort of Ta, T1, and CIS (n=128): 29%
- CIS cohort (n=74): 17%
- Ta/Ta cohort (n=55): 47.1%
Sasanlimab
Sasanlimab is an anti-PD-1 monoclonal antibody that has demonstrated an acceptable safety profile and promising clinical activity in patients with locally advanced or metastatic urothelial carcinoma, within the context of a phase 1 trial.18 The phase 3 CREST study (NCT04165317) Cohort B will evaluate subcutaneous sasanlimab in patients with BCG-unresponsive NMIBC. This is a non-randomized, multicenter, multinational, open-label, phase 3 trial that plans to enroll ̃160 patients with histologically confirmed BCG-unresponsive, high-risk NMIBC (high-grade Ta or T1 tumor or CIS) in two separate cohorts:
- Cohort B1 (n=110): Persistent or recurrent CIS +/- concomitant high-grade Ta/T1 within 12 months of completion of adequate BCG
- Cohort B2 (n=50): Recurrent high-grade Ta/T1 disease within 6 months of completion of adequate BCG therapy or high-grade T1 at 1st evaluation following an adequate induction BCG course

Nivolumab + Linrodostat mesylate
Nivolumab, an anti-PD-1 agent, is currently FDA-approved in the adjuvant setting for patients with muscle invasive bladder cancer in the post-radical cystectomy setting, based on the results of CheckMate 274.20 Indoleamine 2,3-dioxygenase (IDO) is an enzyme modulating tryptophan catabolism and immune responses and promotes cancer progression. Given the increased expression of IDO and PD-L1 in NMIBC tumors, particularly in those that are BCG unresponsive, it is hypothesized that the combination of anti-PD-1 and IDO1 inhibitors may have an additive benefit in this setting.21,22 Linrodostat mesylate, a selective, potent, once-daily IDO1 inhibitor, has demonstrated clinical activity in combination with nivolumab in patients with immunotherapy-naive advanced bladder cancer who received ≥ 1 prior line of therapy (objective response rate: 37%).
CheckMate 9UT, a phase 2, randomized, open-label study is assessing the safety and efficacy of nivolumab +/- linrodostat +/- intravesical BCG in patients with BCG-unresponsive, high-risk NMIBC (CIS +/- papillary disease). Using a novel adaptive-type design, patients will be randomized to one of four treatment arms as follows:
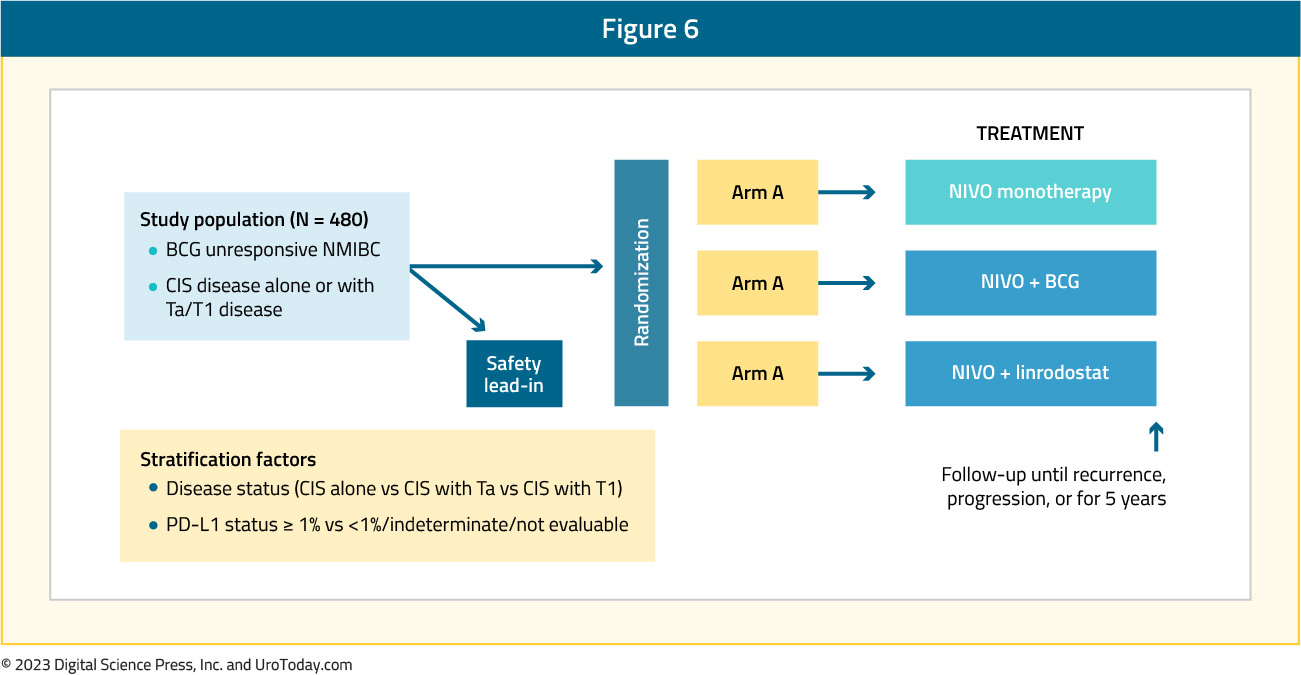
The key exclusion criteria include locally advanced or metastatic bladder cancer, upper urinary tract disease within the prior two years, prostatic urethral disease within one year, and prior immunotherapy. The primary endpoints include the proportion of CIS patients with complete response and the duration of the complete response. Secondary endpoints are progression-free survival and safety.
This global study, started on May 25, 2018, is underway in 14 countries (including the United States, Canada, Russia, Australia, and Brazil), with a target enrollment of 480 patients. The estimated study completion date is September 16, 2026.
Conclusions
Following the FDA approval of pembrolizumab for BCG-unresponsive patients with NMIBC, we have seen the emergence of numerous trials in this disease space evaluating immune checkpoint inhibitors, either alone or in combination with other agents. Based on the currently available data, it appears that short-term (i.e., 3 months) complete response rates with the current agents are modest, with relative short duration of maintained responses. As such, it remains clear that further work in this disease space is of utmost importance.
Published September 2023


