In this context, they are quite effective in relieving bony pain but did not significantly improve survival.1-3 Since then there are several newer agents that are now available. To date, radiopharmaceuticals have only been approved in the metastatic castration resistant prostate cancer (mCRPC), although trials are underway testing several agents in early disease spaces (ie. metastatic hormone sensitive prostate cancer (mHSPC)). This Center of Excellence article will focus on the data leading to FDA approval of radiopharmaceuticals in mCRPC.
Radium-223 Monotherapy
Radium-223 dichloride (radium-223) is a targeted alpha emitter that selectively binds to areas of increased bone turnover in bone metastases and emits high-energy alpha particles of short range (<100 μm). Radium-223 acts as a bone-seeking calcium mimetic and binds into newly formed bone stroma, especially within the microenvironment of osteoblastic or sclerotic metastases.4 Double-stranded DNA breaks result secondary to the high-energy alpha-particle radiation. This results in a potent and highly localized cytotoxic effect in the target areas. Furthermore, the short path of the alpha particles also theoretically minimizes the toxic effects on adjacent healthy tissue, including the bone marrow.4The ALSYMPCA trial was published in 2013 and was a phase 3, randomized, double-blind, placebo-controlled trial that randomized 921 patients in a 2:1 fashion to receive six injections of radium-223 (at a dose of 50 kBq per kilogram of body weight intravenously) or matching placebo. All patients received additional best standard of care. Of note, patients could have received prior docetaxel (57% of included patients). Patients receiving radium-223 had significantly improved median overall survival (14.9 versus 11.3 months; HR 0.70, 95% CI 0.58 to 0.83):
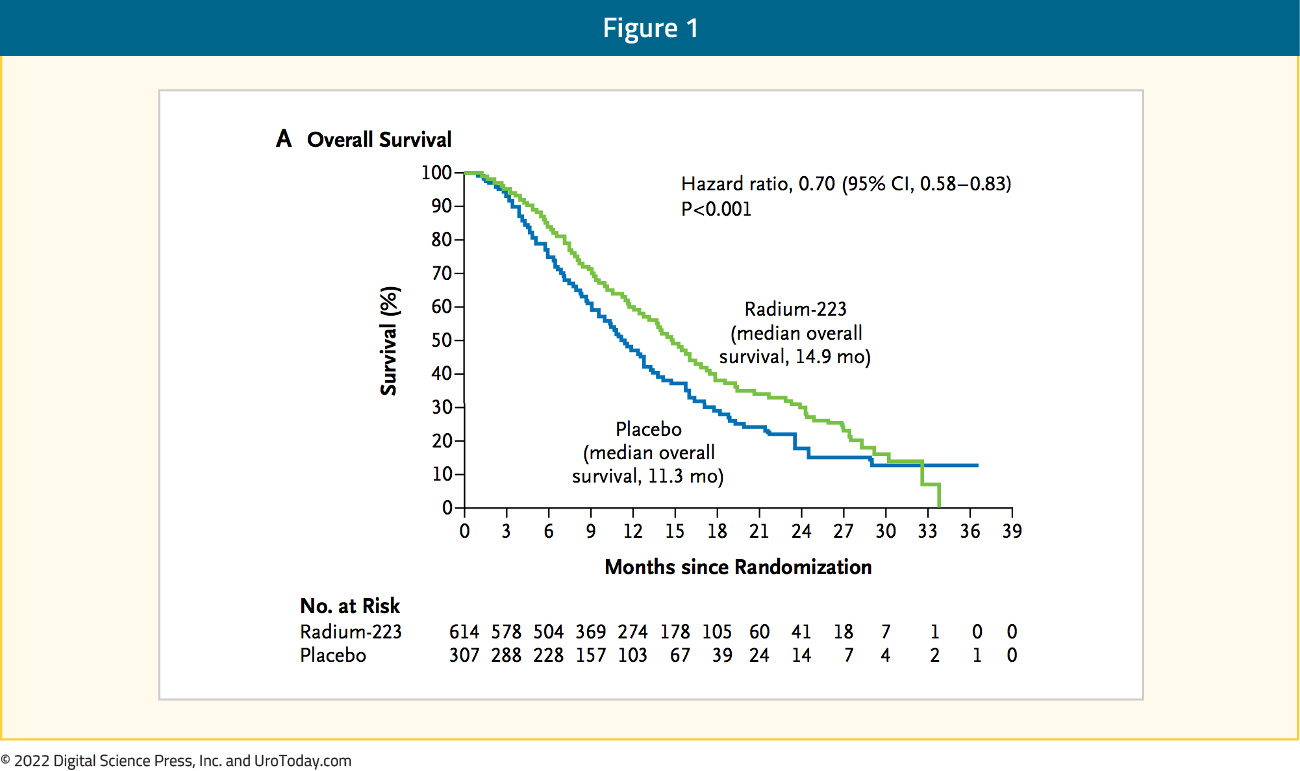
Use of radium-223 was further associated with significantly prolonged time to the first symptomatic skeletal event (15.6 versus 9.8 months, p<0.001), time to increase in the total alkaline phosphatase level (HR 0.17, p<0.001), and time to increase in PSA level (HR 0.64, p<0.001). Grade 3-4 AEs were seen less frequently in the treatment arm (56% versus 62%).4 These results led to the FDA approval of radium-223, however, it is limited by the reliance on the calcium mimetic mechanism of action: as a result, it is only suitable for and approved for patients with bone-only disease. In contrast, alternative approaches to targeting radiopharmaceuticals may allow targeting of soft-tissue disease as well.
PSMA Theranostics
The field of theranostics has witnessed enormous growth over the last few years. Prostate-specific membrane antigen (PSMA) is a transmembrane protein expressed in all forms of prostate tissue, including carcinoma. The PSMA gene is located on the short arm of chromosome 11 in a region that is not commonly deleted in prostate cancer, thus making it highly prevalent in all forms of prostate cancer, including mCRPC. Importantly, PSMA is relatively poorly expressed in other organs (apart from salivary), therefore PSMA targeted theranostic treatment allows for relatively specific targeted therapy. As a result, PSMA-targeted imaging with PSMA-PET/CT has rapidly gained popularity as an imaging modality in prostate cancer. Importantly, PSMA does have an internalization signal that allows for the internalization of the extracellular component into an endosomal compartment.5 This has allowed for the combination of PSMA-617 and the beta-emitter lutetium to deliver targeted ß-particle radiation to PSMA-expressing cells and the surrounding microenvironment.TheraP
After single center series demonstrated the efficacy of Lutetium-177 [177Lu]-PSMA-617 in patients with heavily pre-treated mCRPC,6,7 177Lu-PSMA-617 was evaluated in two large trial settings: TheraP and VISION. Presented initially at ASCO 2020 and since published in The Lancet,8 TheraP was the first randomized study to evaluate 177Lu-PSMA-617 vs cabazitaxel for men with mCRPC after docetaxel. In this open label, phase II trial, 200 men were randomized to either 177Lu-PSMA-617 or cabazitaxel. To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (at least one site with SUVmax≥20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. Overall, 200 patients were randomized 1:1 to 177Lu-PSMA-617 at a dose of 6-8 GBq every 6 weeks for up to 6 cycles of therapy or cabazitaxel 20 mg/m2 every 3 weeks for up to 10 cycles. Patients were stratified based on disease burden and prior anti-androgen therapy. Of note, about 1/3 of patients who had registered for the study (91/291) were ineligible prior to randomization, either because of low PSMA expression or FDG discordant disease. The primary endpoint of this study was a PSA decline of 50% (PSA50) and secondary endpoints included PSA-PFS and OS. After a median follow up of 13 months, 177Lu-PSMA-617 significantly improved PSA-PFS compared with cabazitaxel (HR 0.63, 95% CI 0.46 to 0.86) and had a much higher PSA50 rate (66% vs 37%):

Additionally, there were superior RECIST response rates (33% versus 53%). Regarding the AE/safety profile, Grade 3/4 toxicity was seen in 54% of men on cabazitaxel compared to 35% of patients who received 177Lu-PSMA-617. Rates of thrombocytopenia, dry mouth, and dry eyes were seen more frequently in patients receiving 177Lu-PSMA-617, as expected due to normal PSMA expression in the salivary and lacrimal glands.9
Updated analysis was recently presented at ASCO 2022 after a median follow-up of 36 months and PFS continued to favor the 177Lu-PSMA-617 arm (HR 0.62, 95% CI 0.45 to 0.85). There were no significant differences in restricted mean survival time OS between the two arms (19.1 months in 177Lu-PSMA-617 arm versus 19.6 months in cabazitaxel arm, 95% CI for difference: -3.7 - +2.7). Notably, OS was significantly worse in the 61/90 excluded patients with evaluable data, with restricted mean survival time OS of 11.0 months in these patients despite 48% and 5% eventually receiving cabazitaxel and 177Lu-PSMA-617, respectively.
Importantly, the magnitude of PSMA uptake has emerged as a predictive biomarker: in patients with PSMA SUVmean levels of 10 or greater, the odds of a response to 177Lu-PSMA-617 were substantially higher (OR 12.2, 95% CI 3.4 to 59.0) as compared to those with PSMA SUVmean of less than 10 (OR 2.2, 95% CI 1.1 to 4.5) (p-value for difference = 0.03). This was reflected in superior PSA50 response rates (91% versus 52%) and PSA-PFS HRs (0.45 versus 0.77) for SUVmean ≥ 10 versus SUVmean < 10. Metabolic tumor volume on FDG-PET was another predictive biomarker, with volume ≥200mL portending significantly worse treatment response rates with both 177Lu-PSMA-617 and cabazitaxel.
VISION
Following on the heels of TheraP, VISION is an international, randomized, open-label phase III study evaluating 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with a next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc) and one or two prior lines of taxane chemotherapy (NCT03511664).10 Patients must have had an ECOG performance status of 0-2 and life expectancy of at least 6 months. Importantly, patients must have had PSMA-positive disease on the basis of a central review of 68Ga-PSMA-11 staging scans. PSMA positivity was defined as uptake greater in metastatic lesions than in the liver. Further, they could have no PSMA-negative metastatic lesions. Following enrollment, patients were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks x 6 cycles) plus standard of care or standard of care alone. Standard of care treatments was at the discretion of the treating investigator; however, cytotoxic chemotherapy, immunotherapy, and radium-223 were explicitly excluded. Most patients received alternative androgen-directed therapies while others received palliative radiotherapy and glucocorticoids.
The authors assessed two alternate primary endpoints: (i) rPFS using the Prostate Cancer Working Group 3 (PCWG3) criteria by independent central review and (ii) OS. Secondary endpoints included ORR (RECIST v1.1), disease control rate, time to first symptomatic skeletal event, and safety/AE profile. VISION enrolled 831 patients between June 2018 and October 2019. In keeping with the 2:1 randomization schema, 551 patients were allocated to 177Lu-PSMA-617 + standard of care, and 280 were allocated to standard of care only. Over a median study follow-up of 20.9 months, treatment with 177Lu-PSMA-617 + standard of care significantly improved OS by a median of 4.0 months (median OS: 15.3 vs 11.3 months; HR 0.62, 95% CI 0.52 to 0.74; p < 0.001, one-sided), compared to standard of care alone, in the overall cohort of all randomized patients (n=831):10

Subgroup analyses, presented at ASCO 2022, demonstrated consistent benefits across numerous subgroups, including: including the number of prior novel hormone therapies, taxane regimens, non-taxane regimens and immunotherapies, prior treatment with bone-sparing agents, Radium-223 and PARP inhibitors, and concomitant treatment with novel hormonal therapies, radiation therapy, and bone-sparing agents.
With regards to the other primary endpoint of rPFS, treatment with 177Lu-PSMA-617 + standard of care significantly improved rPFS by a median 5.3 months (median rPFS, 8.7 vs 3.4 months; HR 0.40, 99.2% CI 0.29 to 0.57; p < 0.001, one-sided):

Similar to OS, rPFS subgroup analyses demonstrated a generally consistent effect, though conclusions were limited in many subgroups due to small numbers. In addition to these primary endpoints, the addition of 177Lu-PSMA-617 to standard of care statistically significantly improved all key secondary endpoints, including ORR (29.8% vs 1.7%), disease control rate (89.0% vs 66.7%) and time to first symptomatic skeletal event (median time: 11.5 vs 6.8 months; HR 0.50, 95% CI 0.40 to 0.62). Further, PSA responses (whether defined as a 50% decrease or an 80% decrease) were significantly more common among those treated with 177Lu-PSMA-617 + standard of care. Patient reported outcomes as evaluated by the FACT-P and Brief Pain Inventory (BPI) scores favored the 177Lu-PSMA-617 arm with delays in time to worsening of 7.3 and 11.4 months, respectively. While a higher rate of high-grade (grade 3-5) treatment-emergent AEs were observed with 177Lu-PSMA-617 (28.4% vs 3.9%) at the time of initial reporting, overall therapy was well tolerated. It bears note that treatment exposure was more than three times longer in the 177Lu-PSMA-617 group than in the control group. Adjusted safety analysis, accounting for a longer safety observation due to longer rPFS in patients receiving 177Lu-PSMA-617, revealed a comparable incidence of treatment-emergent AEs between arms. More than 50% of patients with mCRPC were able to receive 5 or 6 cycles (of a planned 6 cycles) of 177Lu-PSMA-617. Grade 3-5 treatment related AEs were seen in 60.4% of patients who received 4 or less cycles of 177Lu-PSMA-617 and 46.4% in those who tolerated 5-6 cycles. In the most recent report, 19 treatment-related mortalities were observed.
Upcoming PSMA-targeted Theranostic Trials
Given the efficacy of 177Lu-PSMA-617 in both the TheraP and VISION trials, there are now several exciting trials that are already enrolling patients or in the development stage. The Study evaluating metastatic castrate-resistant Prostate cancer using 177Lu-PNT2002 PSMA therapy After Second line Hormonal treatment (SPLASH) (NCT04647526) trial is a multicenter, open-label phase III trial that will evaluate 177Lu-PNT2002 in patients with progressive mCRPC after novel hormonal therapies. To date, 177Lu-PNT2002, also known as [Lu-177]-PSMA-I&T, has been administered to more than 300 patients in administrative reports with PSA responses of 50% or greater in 38-59% of patients.11 This trial will include patients with progressive mCRPC who had received only one novel hormonal therapy (in either the HSPC or CRPC setting) but not have received chemotherapy for CRPC (allowed in mHSPC if >1 year) and are unfit or unwilling to receive chemotherapy. In addition, patients had to have high PSMA expression by PSMA PET/CT per BICR. The initial portion of the study will involve a 25-patient dosimetry lead-in. In this phase, patients will receive up to four cycles of 177Lu-PNT2002 at 6.8 GBq every 8 weeks. Following initial safety and preliminary efficacy evaluation during the dosimetry portion of the study, it will continue to the randomization phase in which approximately 390 patients will be randomized in a 2:1 ratio to receive 177Lu-PNT2002 (Arm A) versus enzalutamide or abiraterone (with prednisone or dexamethasone) (Arm B):

This sample size will allow for 90% power to test the alternative hypothesis of a HR ≤ 0.66 at an α of 0.025 for the primary endpoint of rPFS assessed by BICR using RECIST 1.1and PCWG3 criteria. In addition, a number of key secondary endpoints will be examined including ORR, duration of response, PSA response, biochemical PFS, OS, and safety, and tolerability. For patients randomized to Arm B, cross-over to 177Lu-PNT2002 treatment will be offered at the time of BICR-assessed radiologic progression. The SPLASH trial is currently enrolling patients in Canada and the United States, with an estimated primary completion date of March 2023.
Similar to the SPLASH trial, PSMAfore (NCT04689828) is a multicenter, open-label, randomized phase III trial in adult men with progressive mCRPC and confirmed PSMA expression by [68Ga]Ga-PSMA-11 PET/CT. Eligible patients must be taxane-naïve in the metastatic setting and have received one prior novel hormonal therapy and be a candidate for a change in novel hormonal therapy. The authors aim to recruit and randomize 450 patients in a 1:1 fashion to receive 177Lu-PSMA-617 (7.4 GBq i.v. every 6 weeks for 6 cycles) or a change in novel hormonal therapy to either abiraterone or enzalutamide. In both arms, best supportive care is allowed. The primary endpoint is rPFS according to PCWG3-modified RECIST v1.1 criteria:

There are several secondary and exploratory endpoints, including the key secondary endpoint of OS. Participants with blinded independent centrally confirmed radiographic progression in the novel hormonal therapy arm can crossover to the 177Lu-PSMA-617 arm. The primary efficacy and safety analyses are planned with a sample of 156 rPFS events which will allow for a 95% power to detect a HR of 0.56 for rPFS with an overall one-sided significance level of 0.025. OS will be further analysed once approximately 297 deaths have been observed. The first participant was enrolled on study on June 15, 2021, and patients are currently being recruited in a number of countries in North American and Europe. The estimated study completion date is December 2024.
Numerous other clinical trials evaluating PSMA-targeted radioligand therapy are ongoing both among patients with mCRPC and earlier in the disease process. Many of these studies will examine combination therapy with PSMA-targeted radioligand therapy with other agents. These will be discussed in further detail in a related article examining combination therapy in mCRPC.
- EnzaP – randomized phase 2 trial of enzalutamide with 177Lu-PSMA-617 vs. enzalutamide alone in patients with mCRPC (NCT04419402)
- Randomized phase 2 trial of 177Lu-PSMA-617 vs. docetaxel for PSMA PET-positive mCRPC (NCT04663997)
- PSMAddition – randomized phase 3 trial of 177Lu-PSMA-617 + standard of care vs. standard of care alone for mHSPC (NCT04720157)
- Phase 1 dose-escalation of fractionated 177Lu-PSMA-617 for mCRPC (NCT03042468)
- UpFrontPSMA – randomized phase 2 trial of 177Lu-PSMA-617 followed by docetaxel vs. docetaxel alone for newly diagnosed high volume mHSPC (NCT04343885)
- LuTectomy – Phase 1/2 trial of 177Lu-PSMA-617 prior to prostatectomy (NCT04430192)
- LuPARP – Phase 1 trial of 177Lu-PSMA-617 with Olaparib for metastatic castration-resistant prostate cancer (NCT03874884)
Written by:
- Rashid Sayyid, MD MSc, University of Toronto, Toronto, ON
- Zachary Klaassen, MD MSc, Medical College of Georgia, Augusta, Georgia, USA


