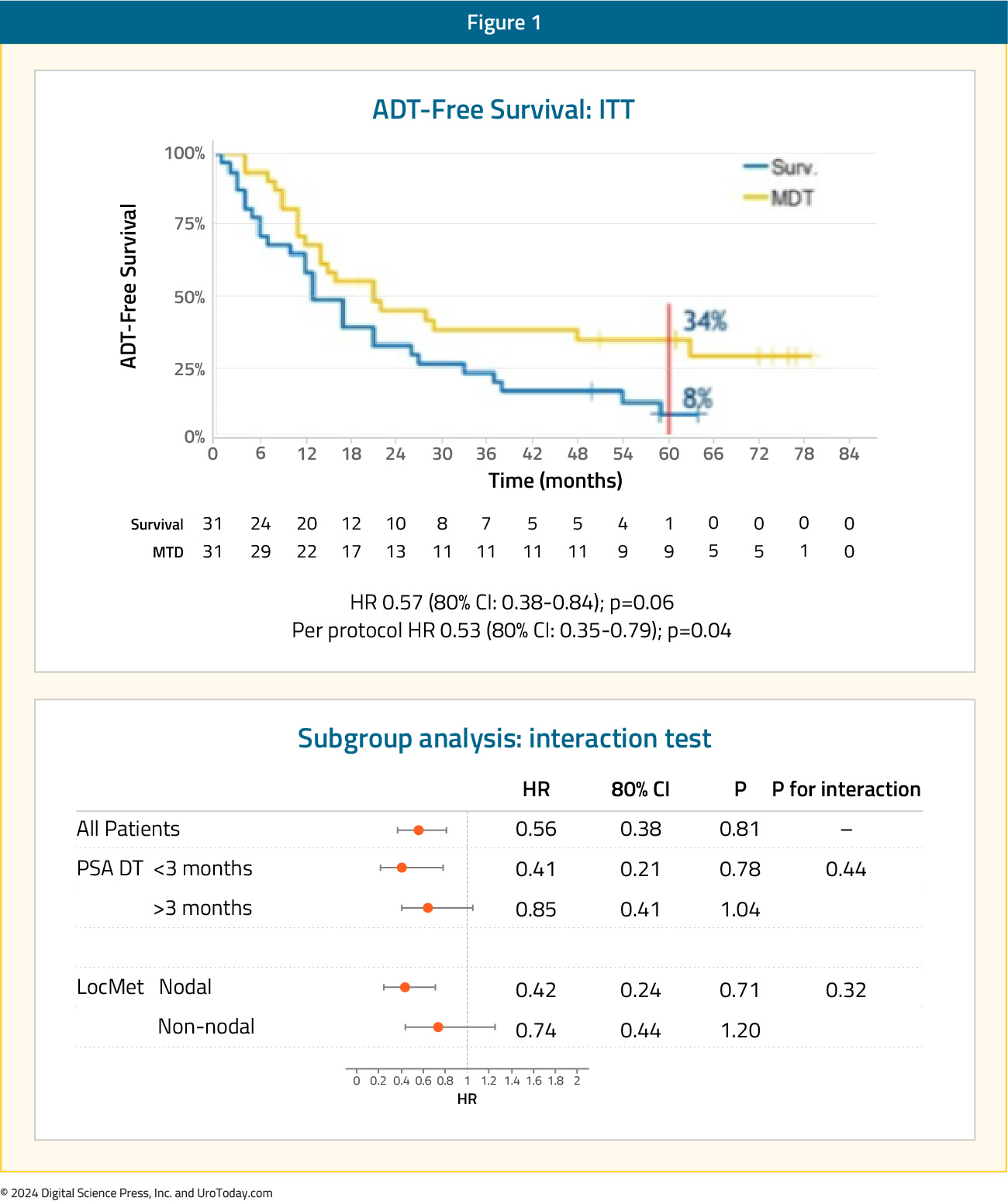
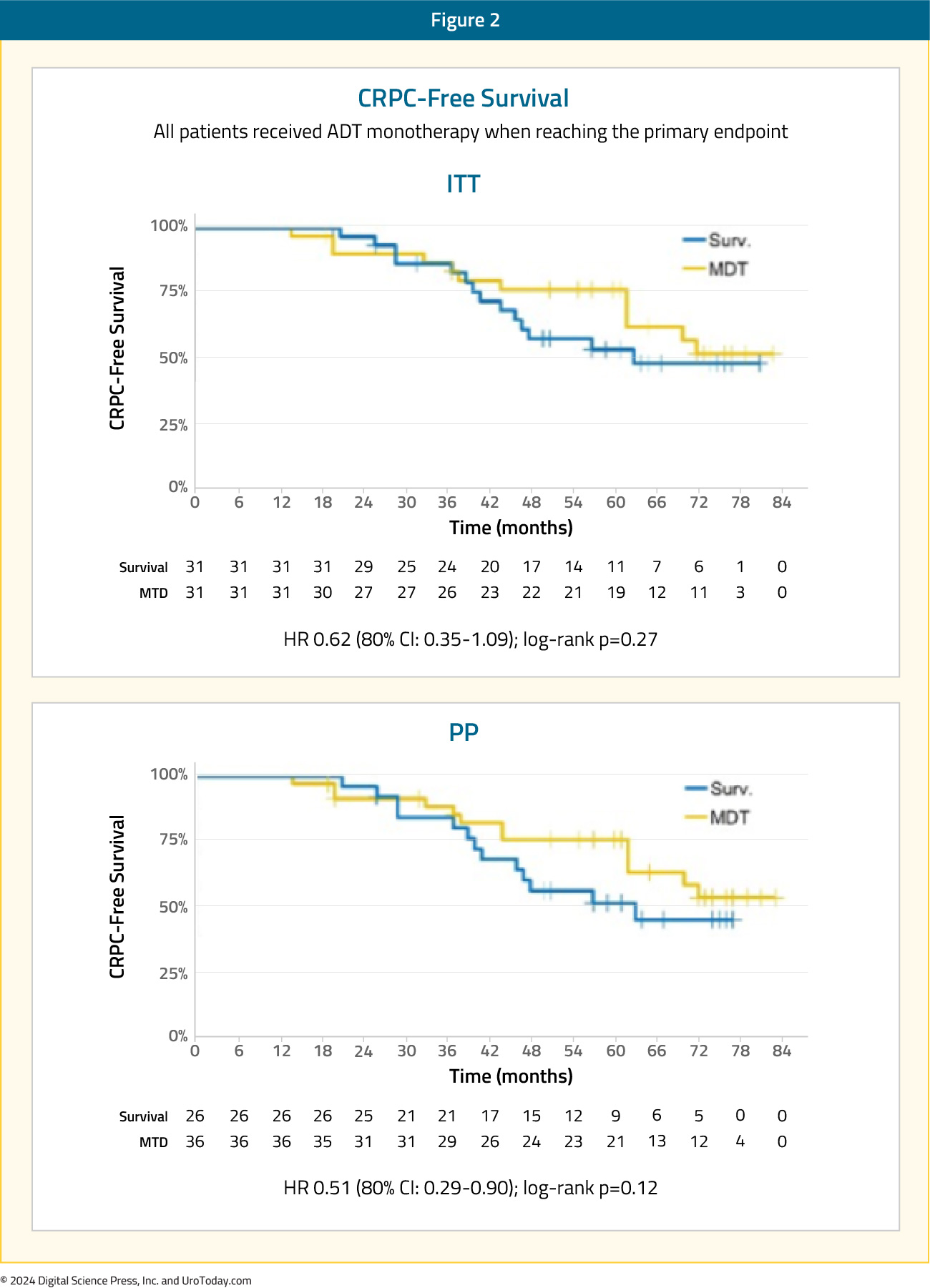
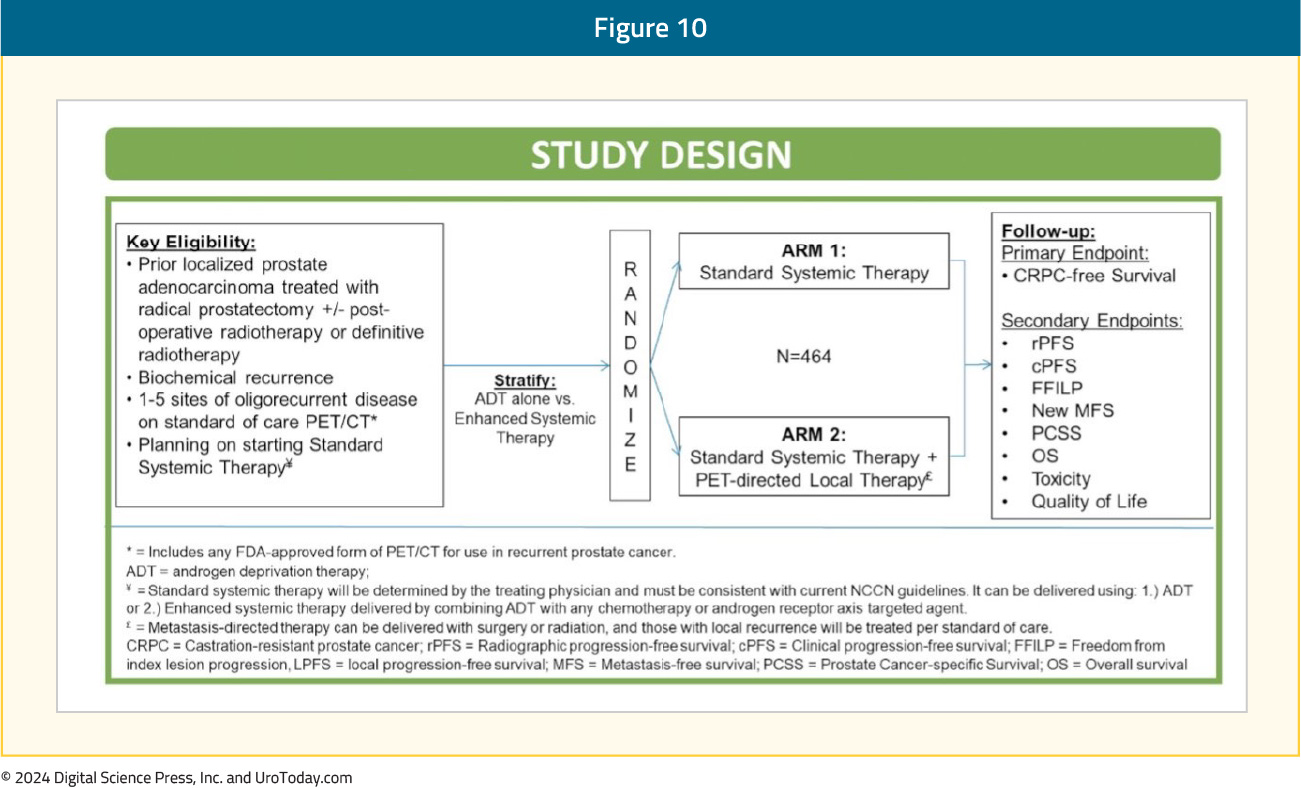
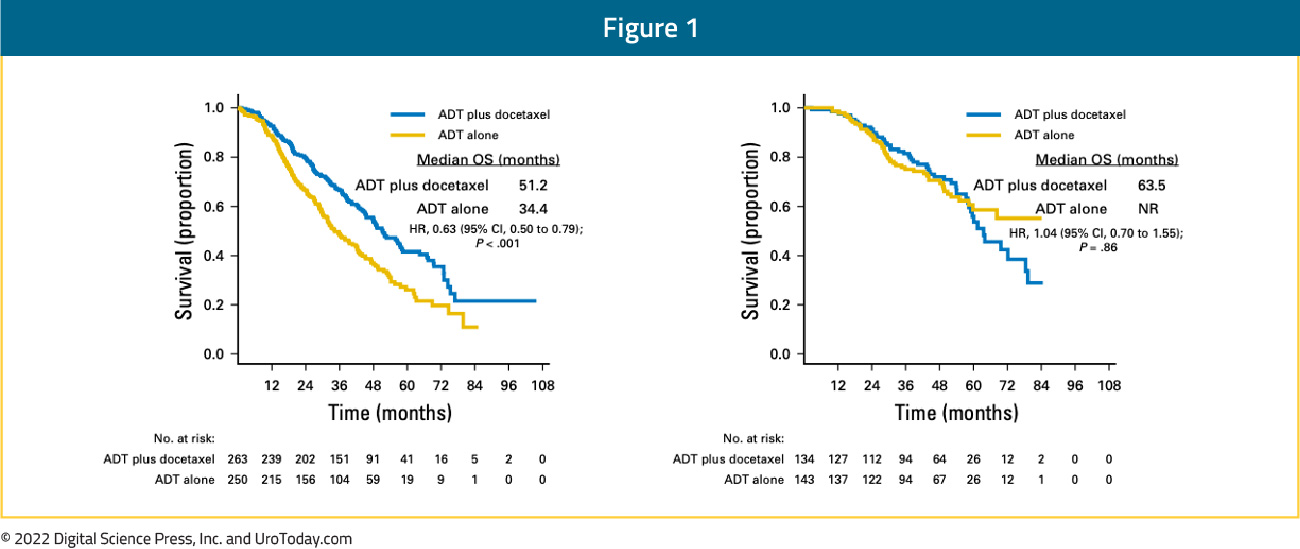
Introduction
Since 1941, the backbone of treatment for advanced prostate cancer has been androgen deprivation therapy (ADT). However, treatment advancement remained relatively stagnant until the last decade, when we saw the emergence of several doublet and triplet therapy options, using ADT as the backbone of treatment, leading to an overall survival (OS) advantage versus ADT alone.
Figure 1: The current landscape of FDA combination approvals in Metastatic Hormone Sensitive Prostate Cancer (mHSPC)1-12
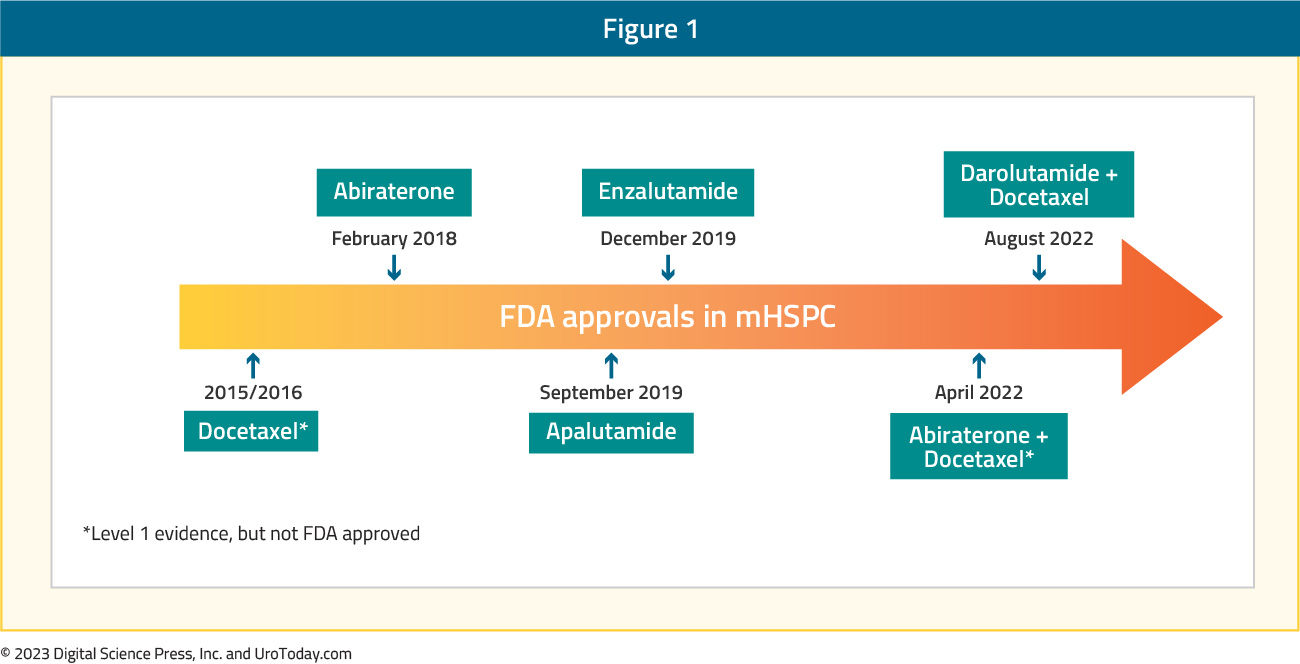
Thus, this has changed the standard of care for treatment intensification for these men. This article will focus a discussion on the implementation of treatment for Metastatic Hormone Sensitive Prostate Cancer (mHSPC) in North America, specifically highlighting the landscape and challenges of treatment intensification, and the importance of disease volume and timing of metastasis for selecting the optimal treatment in the mHSPC disease space.
The Enigma of (Lack of) Treatment Intensification
Despite the approval and availability of multiple mHSPC treatment intensification strategies, there remains a clear underutilization of these combination strategies in real-world practice. The following discussion will highlight several of the key studies looking at contemporary treatment intensification across several North American jurisdictions.
Ryan et al. reported treatment utilization trends of mHSPC patients between January 2014 and July 2019 from two large U.S databases: (i) Optum’s de-identified Clinformatics Data Marta Database (COM/MA), which includes claims from commercial and Medicare Advantage plans for 13 million people across the United States and (ii) Centers for Medicare & Medicaid Services-sourced Medicare Fee-for-Service (FFS) Research Identifiable Files Sample.13 A total of 19,841 mHSPC patients (6,517 COM/MA and 13,324 Medicare-FFS) were identified with a median follow up of 9.6 – 10.5 months. Notably, 38% of COM/MA and 48% of Medicare-FFS patients remained untreated or deferred treatment during the study period, whereas 45% and 46%, respectively, were treated with first line ADT monotherapy only. Abiraterone acetate or docetaxel was used as first line therapy in 13% (COM/MA) and 2%
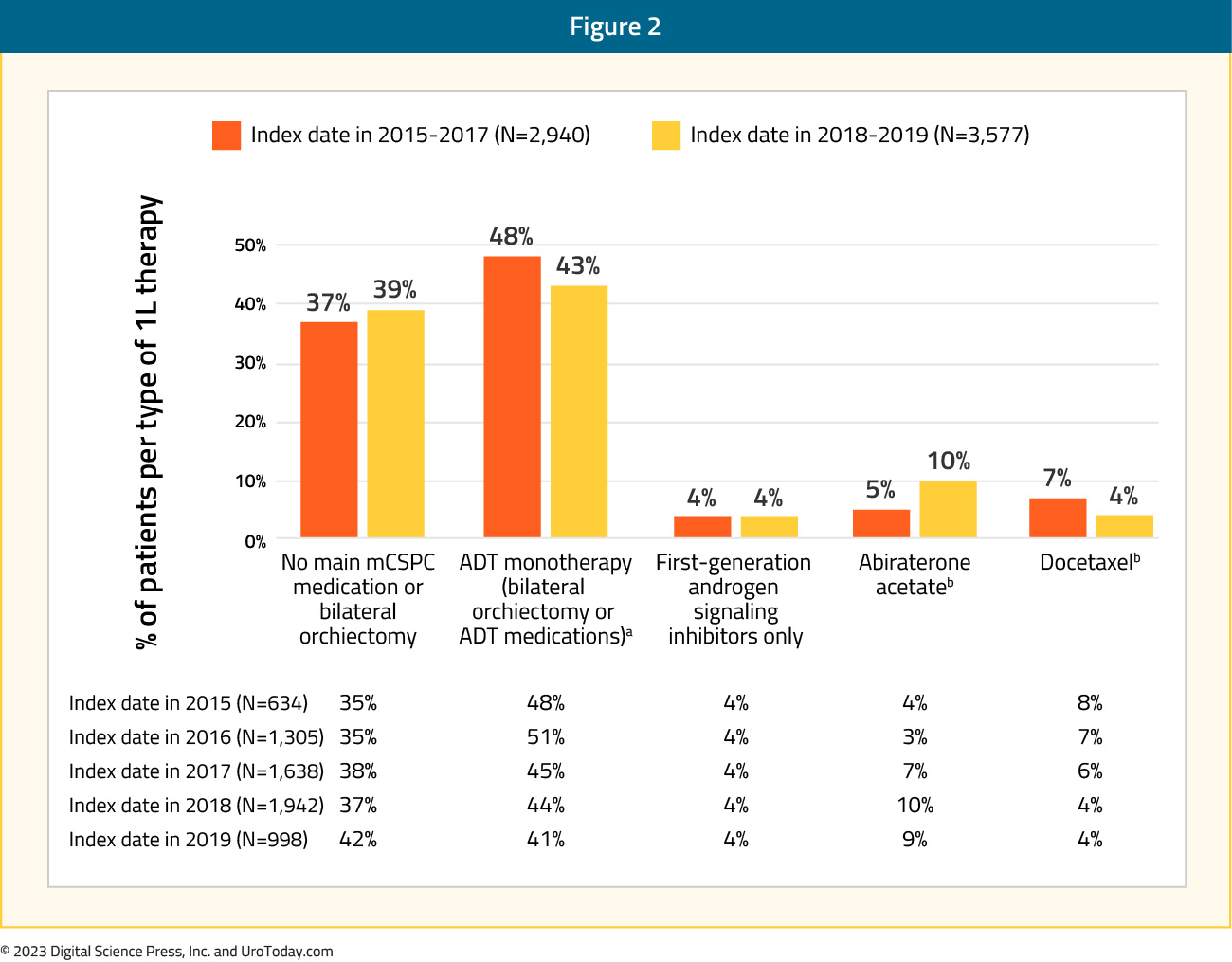
(Medicare-FFS) of patients. Approximately 43% and 38% of patients, respectively, only received ADT monotherapy (with or without a first-generation androgen signaling inhibitors) during the entire mHSPC period despite the availability of other more potent therapies. It is important to note that apalutamide and enzalutamide were added to evidence-based guidelines as mHSPC treatments after the end of the study period (July 31, 2019) and were not considered as mHSPC therapy in the current study. When stratified by year of index date, in the COM/MA database, treatment with first line ADT monotherapy decreased numerically from 48% to 43% among patients diagnosed with mHSPC in 2015 – 2017 versus 2018 – 2019. While the overall use of abiraterone or docetaxel remained similar in the two periods (12% - 14%), the relative use of abiraterone acetate increased among patients diagnosed in 2018 - 2019 (10%) versus 2015 - 2017 (5%), whereas the use of docetaxel decreased in 2018 - 2019 (4%) compared with 2015 – 2017 (7%).
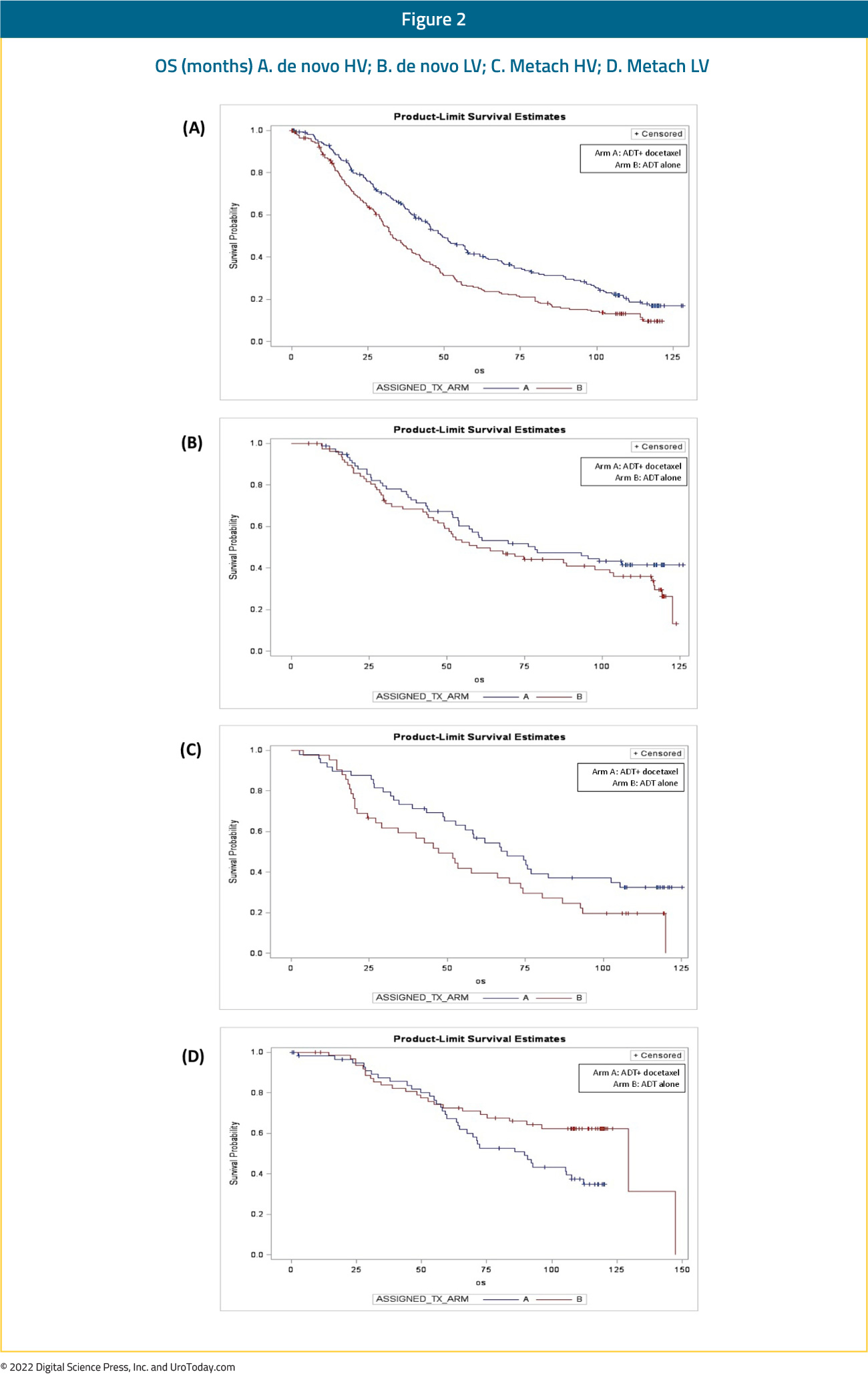
Figure 2: First line therapy in patients with mHSPC by year of index data in the Clinformatics Data Marta Database13
Analysis from the Veterans Health Administration (VHA) database was reported by Freedland et al.14 for 1,395 mHSPC patients treated between April 2013 and March 2018. Across the five-year study period, 63% of patients received ADT alone as first line treatment while ADT + non-steroidal anti-androgen was used in 24%, ADT + docetaxel in 8%, and ADT + abiraterone for the remaining 5%. Treatment trends over time did demonstrate an overall decrease in ADT only (66% to 60%) or ADT + non-steroidal anti-androgen (31% to 17%) utilization between 2014 and 2017-2018, with a corresponding increase in utilization of ADT+ docetaxel (3% to 9%) and ADT + abiraterone (1% to 15%). Nonetheless, despite increased adoption of treatment intensification in VHA mHSPC patients, there remains a clear underutilization of appropriate treatment intensification.
Figure 3: Treatment trends over time among patients with mHSPC in the Veterans Health Administration14
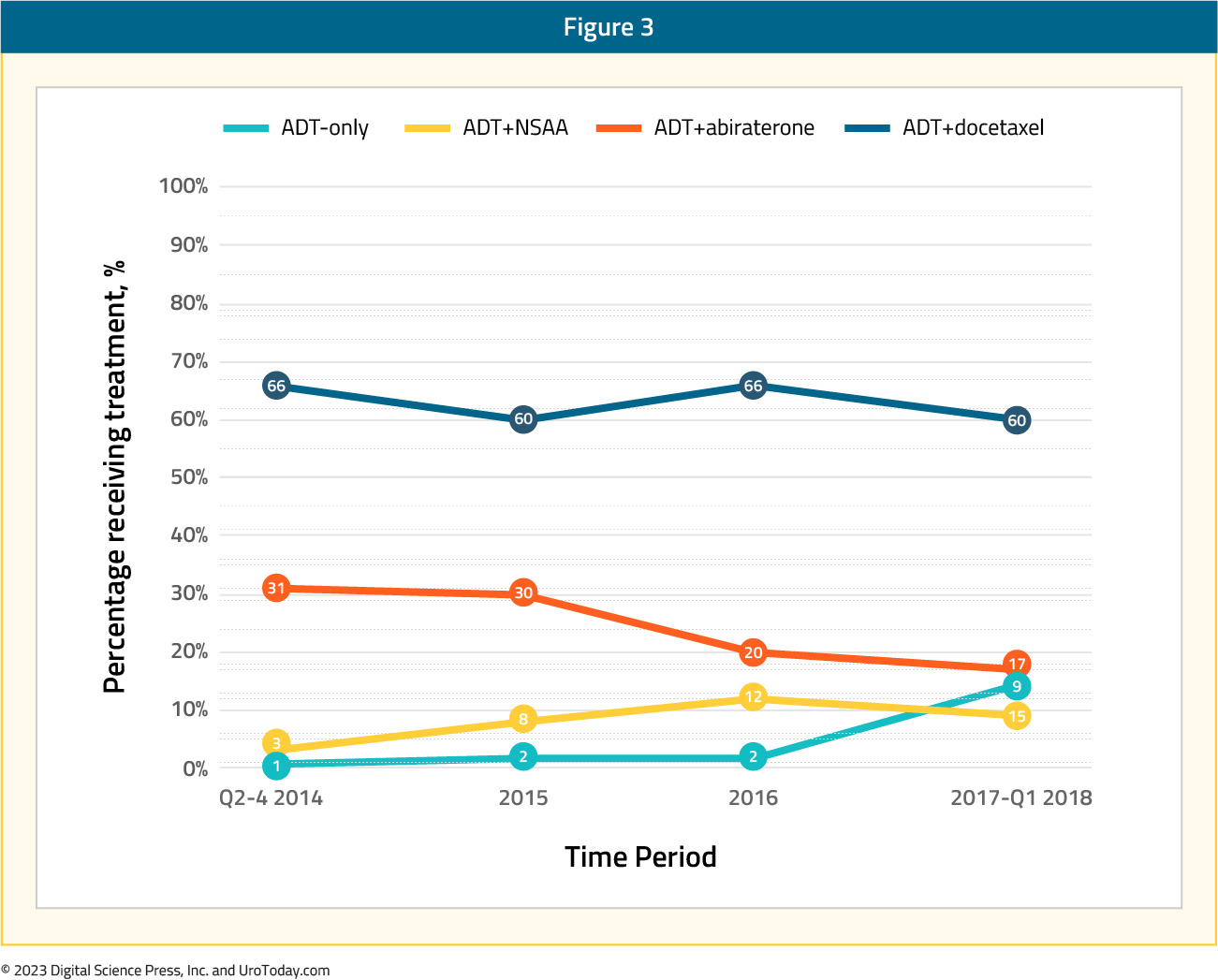
A comparison of patients in the four treatment groups demonstrated that patients receiving ADT and docetaxel, compared to the ADT only cohort, were younger (65.8 versus 73.4 years) and had fewer comorbidities (National Cancer Institute comorbidity score 1.1 versus 1.5), but had greater disease burden in terms of higher PSA (338.1 ng/ml versus 256.4 ng/ml) and overall metastatic burden. ADT + abiraterone patients were older (75.3 versus 73.4 years), generally had fewer cardiovascular comorbidities and lower PSA (238.3 versus 256.5 ng/ml) but had increased metastasis (other sites including bone: 80% versus 73%).
A combined analysis from the VHA and Medicare database was presented at ESMO 2022. The authors identified 33,641 and 5,561 men in the Medicare and VA cohorts, respectively. Similar to the prior report by Freedland et al., 14 the authors demonstrated that the proportion of patients receiving treatment intensification with novel hormonal therapy or docetaxel increased over time, although by 2018/2019, still less than one third of patients with mHSPC received first line ADT plus docetaxel or ADT plus a novel hormonal agent.
Figure 4: Utilization of first line treatment intensification over time for mHSPC patients in the VHA and Medicare databases

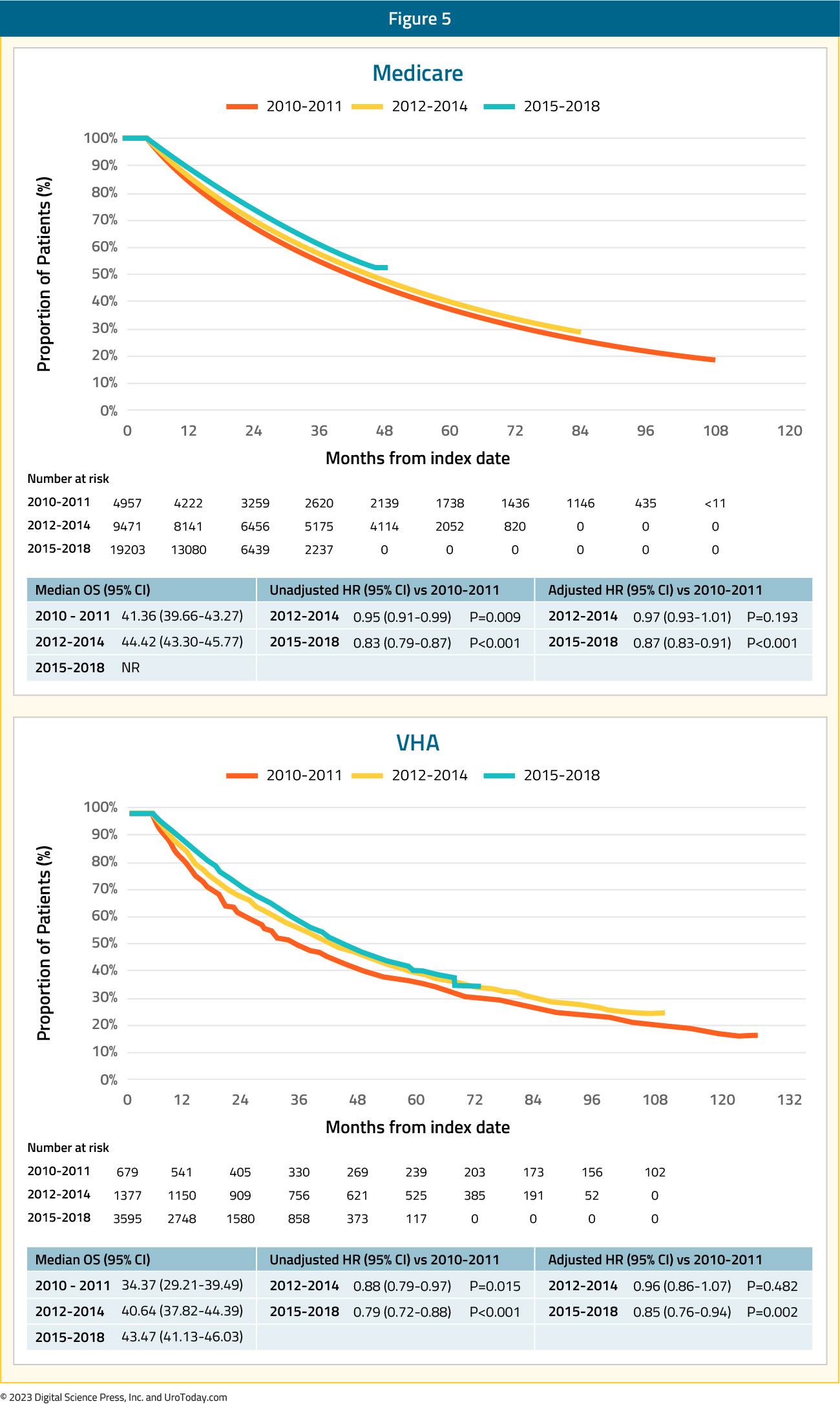
While there were changes in treatment approaches between 2010 and 2014, the authors did not find any changes in overall survival in 2012–2014 compared with 2010–2011, though there was improvement in overall survival by 12% and 15% in the Medicare and VA cohorts, respectively, in 2015–2018/2019 versus 2010–2011, after adjusting for baseline characteristics, suggesting that diffusion of these intensified treatment approaches provides a population-level survival benefit.
Figure 5: Overall survival for mHSPC patients in the VHA and Medicare databases
A Medicare analysis of treatment intensification trends among racial minorities was presented by Freedland et al. at ASCO 2021. Compared to White, non-Hispanic men, Black men were less likely to receive treatment intensification (ADT + docetaxel or novel hormonal therapy) during 2010-2014 (2.6% versus 3.2%), 2015-2016 (8.7% versus 10.4%), and 2017 (14.2% versus 15.1%).
Using provincial data from Ontario, Canada, Wallis et al.15 identified 3,556 patients diagnosed with de novo mHSPC between 2014 and 2019. Of note, 78.6% of patients received ADT alone (with or without an anti-androgen), 11.2% received treatment intensification with docetaxel, 1.5% received abiraterone acetate and prednisone, with the remaining 8.7% receiving a “non-ADT” regimen. Patients receiving docetaxel were comparatively younger (mean age 72.6 years) and healthier (mean Charlson Comorbidity Index score of 0.15). The median PSA at diagnosis was lower among patients who received conventional ADT (88 ng/mL) compared with ADT intensification regimens (121 ng/mL and 152 ng/mL for the abiraterone and docetaxel cohorts, respectively). A time-stratified analysis representing the uptake of ADT intensification regimens before and after the pivotal 2017 LATITUDE trial,8 demonstrated that abiraterone acetate plus prednisone prescriptions increased from 0.5% to 3% in the pre- versus post-LATITUDE period, respectively, whereas docetaxel treatment dropped from 12% to 10%. As was reported by Ryan et al.13, these data suggest that the pivotal data from LATITUDE and STAMPEDE resulted in a substitution of intensification approach (from docetaxel to abiraterone) rather than a broadening of the patient population receiving treatment intensification.
Given the underwhelming utilization of treatment intensification for mHSPC patients in the real-world setting, barriers to improved adoption need to be further understood. At ASCO 2022, Freedland et al. provided the first granular assessment as to reasons for or against treatment intensification. This study examined data from medical charts of patients initiating mHSPC treatment from July 2018 to November 2021 based on a retrospective review of multiple US academic/community practices. This was a survey of oncologists and urologists who treated these patients to provide reasons for treatment choices, including PSA goals and explicit reasons for not prescribing novel hormonal agents. This analysis included 621 patients who were treated by 65 oncologists and 42 urologists. In the first line setting, most mHSPC patients received ADT ± non-steroidal anti-androgen alone (69%), while treatment intensification rates with ADT + novel hormonal agent (26%) or ADT + chemohormonal therapy (4%) were low. Following the initial treatment course, an additional 166 patients (27%) received subsequent treatment intensification while still castration-sensitive, prior to progression to castration resistant disease.
When the physicians were queried about reasons for not using novel hormonal agents, the most frequently cited reasons were:
- “Novel hormonal therapy would need to have a better/more tolerable side effect profile/fewer adverse events than my chosen regimen” (38%)
- “I would need to have seen clinical trial evidence of survival improvements on novel hormonal therapies including a wider range of prostate cancer patients” (31%)
- “Novel hormonal therapies would need to be reimbursed by patients’ insurance” (26%)
Figure 6: Reasons given by providers for not using novel hormonal therapy

Regarding treatment goals for PSA response, physicians more frequently reported a relative reduction than an absolute PSA reduction (85% versus 51%). Oncologists considered a median PSA reduction of 50% (IQR 25-75%) adequate versus 75% (IQR 50-90%) among urologists. Urologists were more likely to utilize treatment intensification in the first line setting or subsequently in patients who were still castration-sensitive (p < 0.01). Furthermore, physicians who aimed for deeper PSA reductions of 75-100% were more likely (OR: 1.63, p = 0.034) to provide treatment intensification in the first-line setting compared with physicians with less aggressive PSA goals (0 – 49%). The authors concluded that physician survey results suggest that perceptions of tolerability and lack of efficacy and financial considerations affect novel hormonal therapy use. In practice, non-guideline driven PSA reduction goals are associated with low rates of treatment intensification, and these results clearly demonstrate the need for further medical education.
Treating Implementation: Using Disease Volume and Timing of Metastasis to Guide Treatment Selection
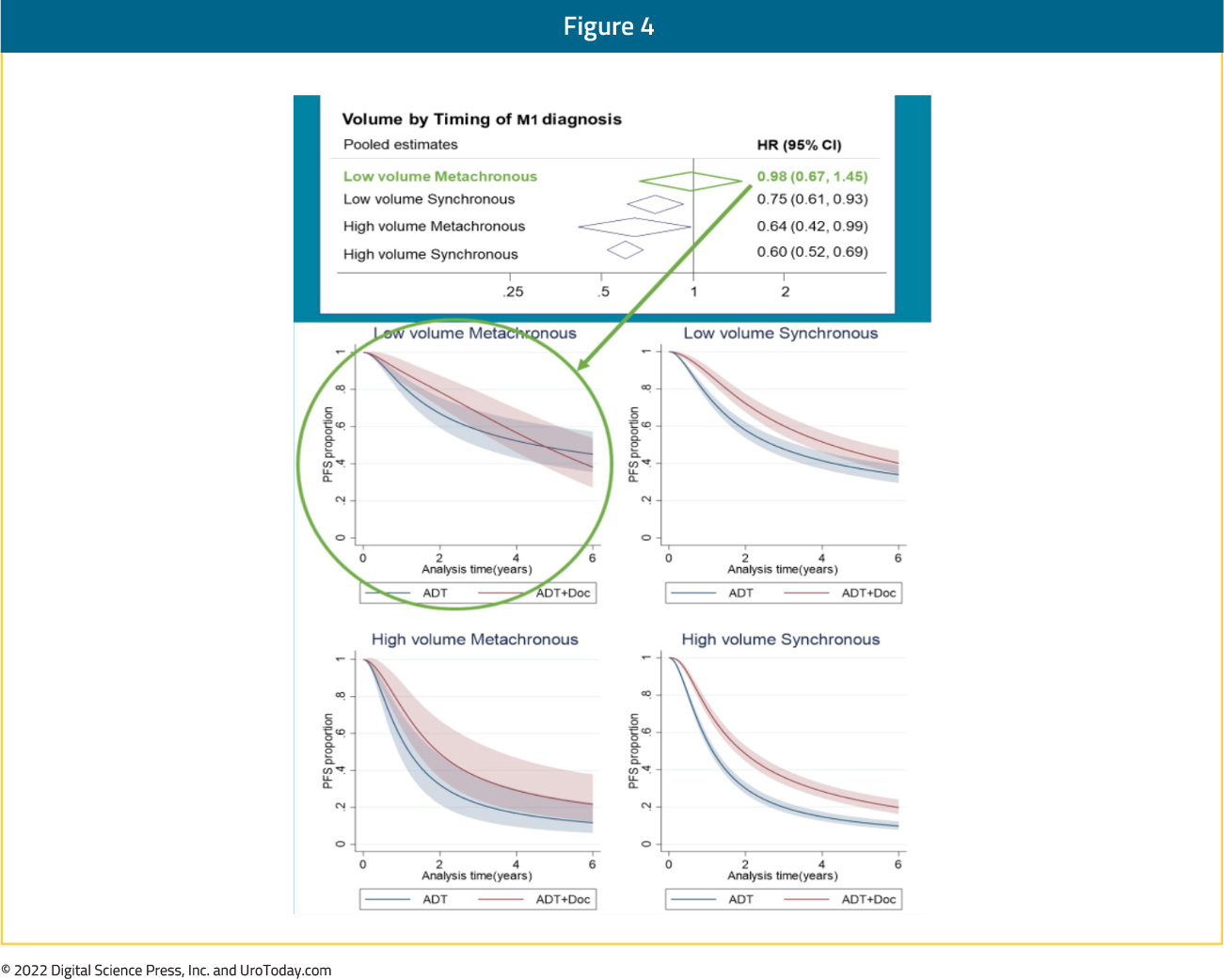
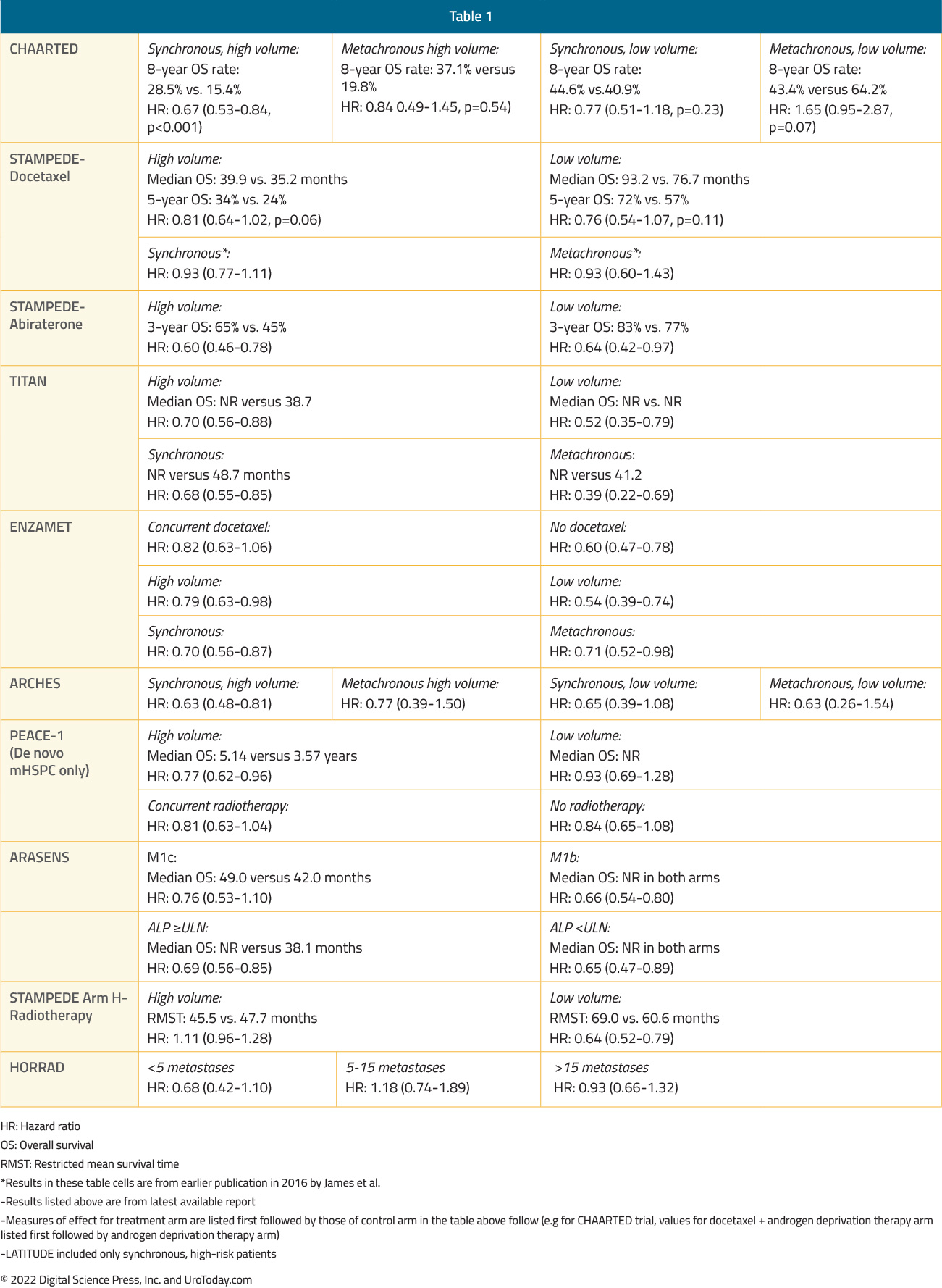
Patients with mHSPC at the time of diagnosis are defined as having de novo or synchronous metastatic disease. Additionally, there is a subset of men initially diagnosed with non-metastatic disease, many of whom had received prior definitive local treatment, who will have progression to a metastatic state prior to development of castration resistance; this is known as metachronous mHSPC. This distinction between synchronous (i.e. de novo) and metachronous presentations is of utmost clinical importance given the known differences in underlying genomic mutational profiles and prognoses, influencing the subsequent choice of treatment intensification.16 These two cohorts can be further subdivided based on the volume of metastatic disease at presentation: low and high volumes. The CHAARTED high-volume criteria have been widely adopted in clinical practice, with high volume patients defined as follows: presence of visceral metastases or ≥4 bone lesions with ≥1 beyond the vertebral bodies and pelvis.17
As such four distinct subgroups become clinically relevant (median OS per CHAARTED and GETUG-15 among men receiving ADT alone, i.e., the control groups in these trials):
- Synchronous and high volume: 3 years
- Synchronous and low volume: 4.5 year
- Metachronous and high volume: 4.5 years
- Metachronous and low volume: ~8 years
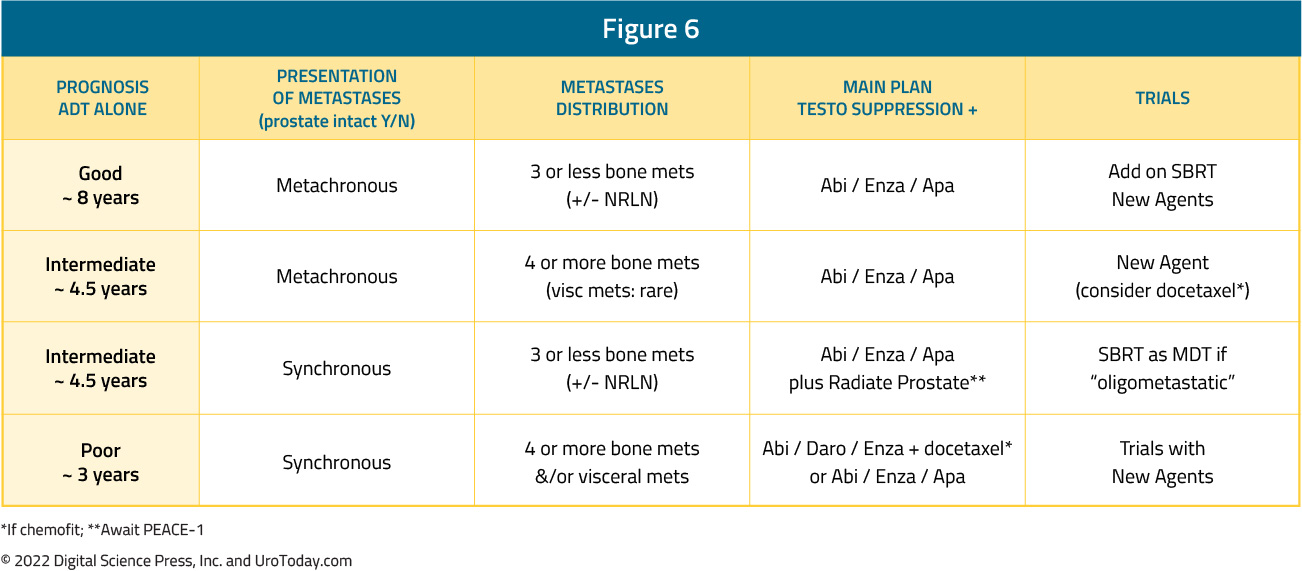
While early, aggressive treatment intensification with triplet regimens, with or without primary radiotherapy, may seem attractive in this cohort of patients to maximize survival outcomes, the reality is that such “maximal” treatment intensification is unnecessary in the majority of these patients. Furthermore, treatment toxicity, both from a pathophysiologic and financial standpoint, must be considered in these patients. As such, a nuanced approach to the treatment of such patients, guided by the aforementioned four presentations (synchronous high volume, synchronous low volume, metachronous high volume, and metachronous low volume) is needed. Arguably, over the next several years, particularly until reliable biomarkers become available, this will be how most clinicians implement treatment for mHSPC patients in North America.
Synchronous High Volume mHSPC
Based on the results from PEACE-118 and ARASENS10, as well as subgroup analysis from ENZAMET3, it appears that this patient cohort, particularly those who are chemotherapy-fit, are most likely to benefit from triplet therapy with docetaxel + androgen receptor pathway inhibitor (ARPI) + ADT.
ADT + Docetaxel + Abiraterone
The PEACE-1 trial18 employed a 2x2 design to assess, (separately and combined) the impact of the addition of abiraterone + prednisone +/- radiation therapy to standard of care therapy in men with de novo mHSPC. Among patients with high volume disease, the addition of abiraterone + prednisone to standard of care resulted in a 53% improvement in rPFS with a median rPFS of 1.6 years in the standard of care arm and 4.1 years in the standard of care plus abiraterone + prednisone arm (HR: 0.47, 95% CI: 0.36 to 0.60). The addition of abiraterone + prednisone to standard of care in patients with low volume disease still resulted in a 42% improvement in rPFS with median rPFS of 2.7 years on the standard of care arm versus not yet reached in the standard of care plus abiraterone + prednisone + ADT arm (HR: 0.58, 95%: CI 0.39 to 0.87). With regards to overall survival in patients with a de novo presentation, a benefit was seen mainly in those with high-volume disease (median overall survival 5.1 versus 3.6 years; HR: 0.77, 95% CI: 0.62 to 0.96), with a marginal, non-significant improvement in those low volume de novo disease (median overall survival not reached; HR: 0.93, 95% CI: 0.69 to 1.28). The overall survival data is immature for the low volume patients due to a small number of events.
Notably, 81% of patients in the ADT plus docetaxel standard of care control arm subsequently received a next generation hormonal therapy at the time of disease progression. This suggests that early intensification with the addition of abiraterone + prednisone to standard of care therapy results in improvement in rPFS and OS compared to sequential therapy.
ADT + Docetaxel + Darolutamide
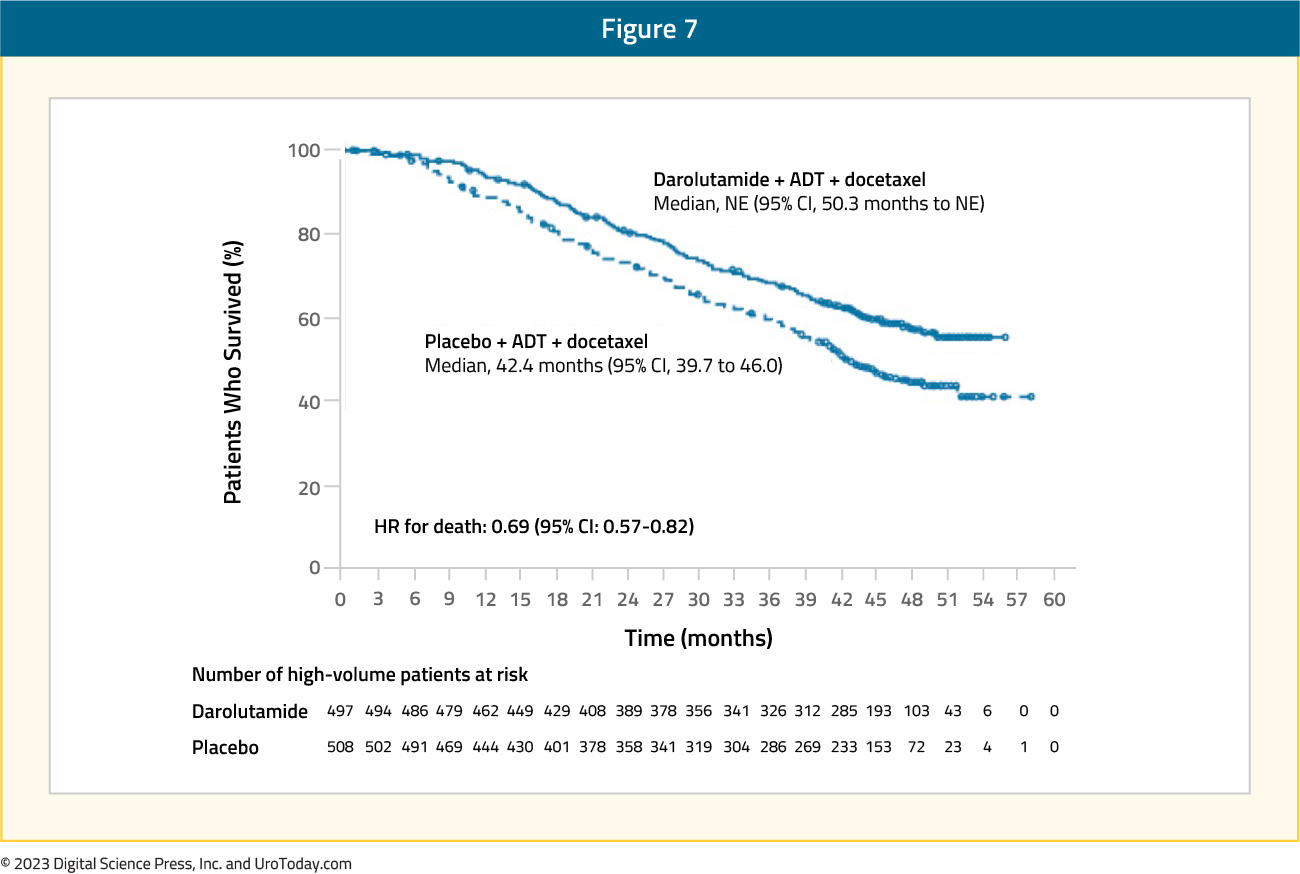
The ARASENS trial evaluated the addition of darolutamide to standard of care therapy consisting of ADT + docetaxel versus ADT + docetaxel alone.10 Darolutamide + ADT + docetaxel prolonged overall survival for high volume mHSPC (HR 0.69, 95% CI 0.57-0.82).19
Figure 7: Overall survival of darolutamide + ADT + docetaxel vs ADT + docetaxel for high volume disease patients in the ARASENS trial
ADT + Enzalutamide vs ADT
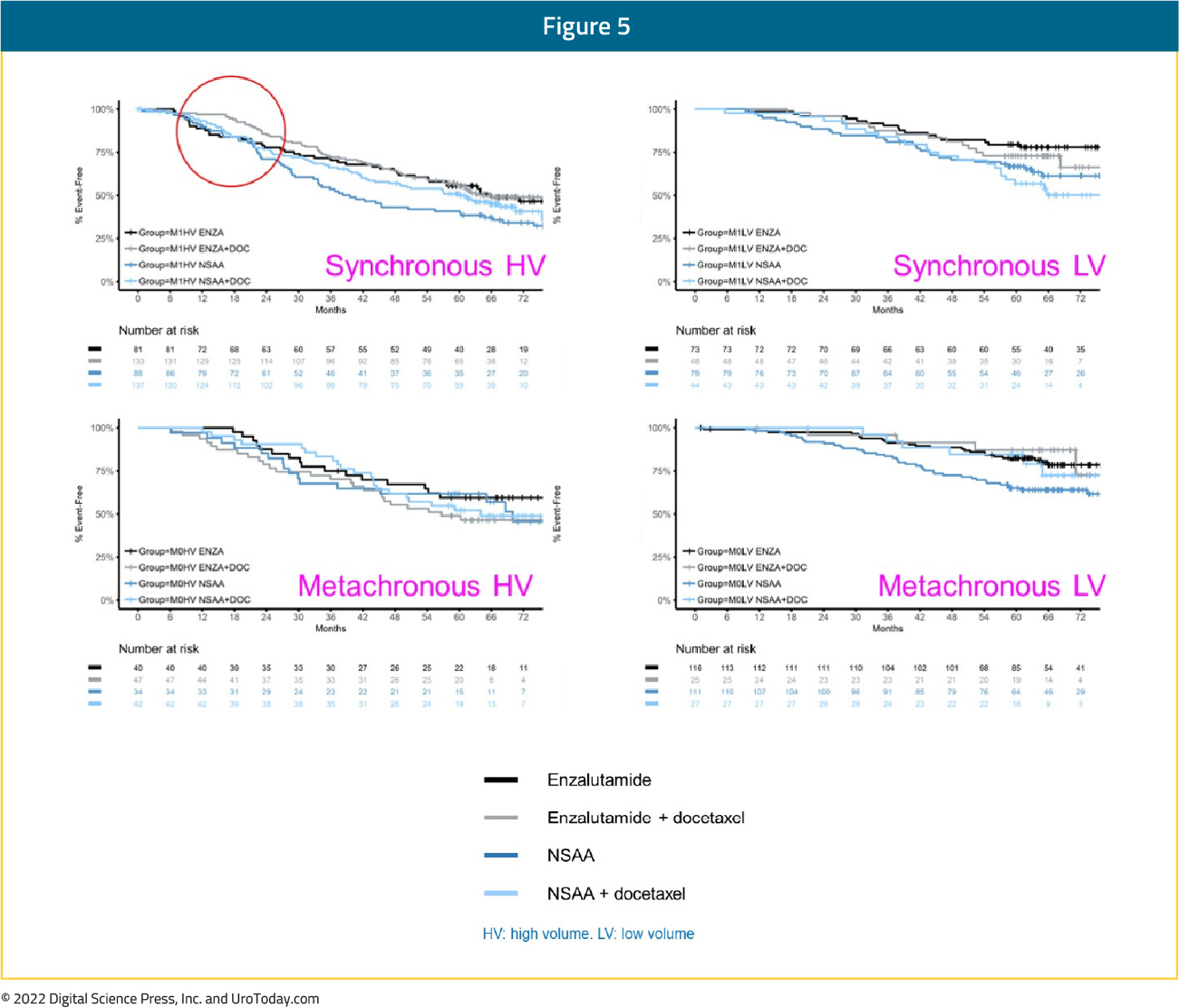
While the ENZAMET trial was designed to compare the combination of ADT + enzalutamide versus ADT + standard nonsteroidal antiandrogen, the study design allowed for previous/concurrent use of docetaxel. In this trial, six cycles of docetaxel were given to 65% of patients in the enzalutamide group versus 76% in the standard of care group. Updated results of the ENZAMET trial were published in 20234, with survival outcomes stratified by disease volume (high versus low) and presentation (synchronous versus metachronous). Based on these subgroup analyses, patients with synchronous, high-volume mHSPC had a clinical benefit, albeit not statistically significant, for ADT + enzalutamide versus ADT + standard nonsteroidal antiandrogen whether they were planned to have docetaxel (HR: 0.79, 95% CI: 0.57 to 1.10) or in the intention to treat analysis (HR: 0.70, 95% CI: 0.47 to 1.04).
Figure 8: Overall survival in ENZAMET for patients with synchronous high volume mHSPC

Results from these three trials provide strong evidence to support the use of a triplet regimen approach in patients with synchronous, high volume mHSPC. It bears note, however, that routine use of docetaxel may not be feasible in patients with contraindications to taxane therapy, including poor performance status, blood dyscrasias, and peripheral neuropathy. Such patients would likely benefit from ARPI addition to standard ADT.
Synchronous Low Volume mHSPC
ADT + Docetaxel + ARAT
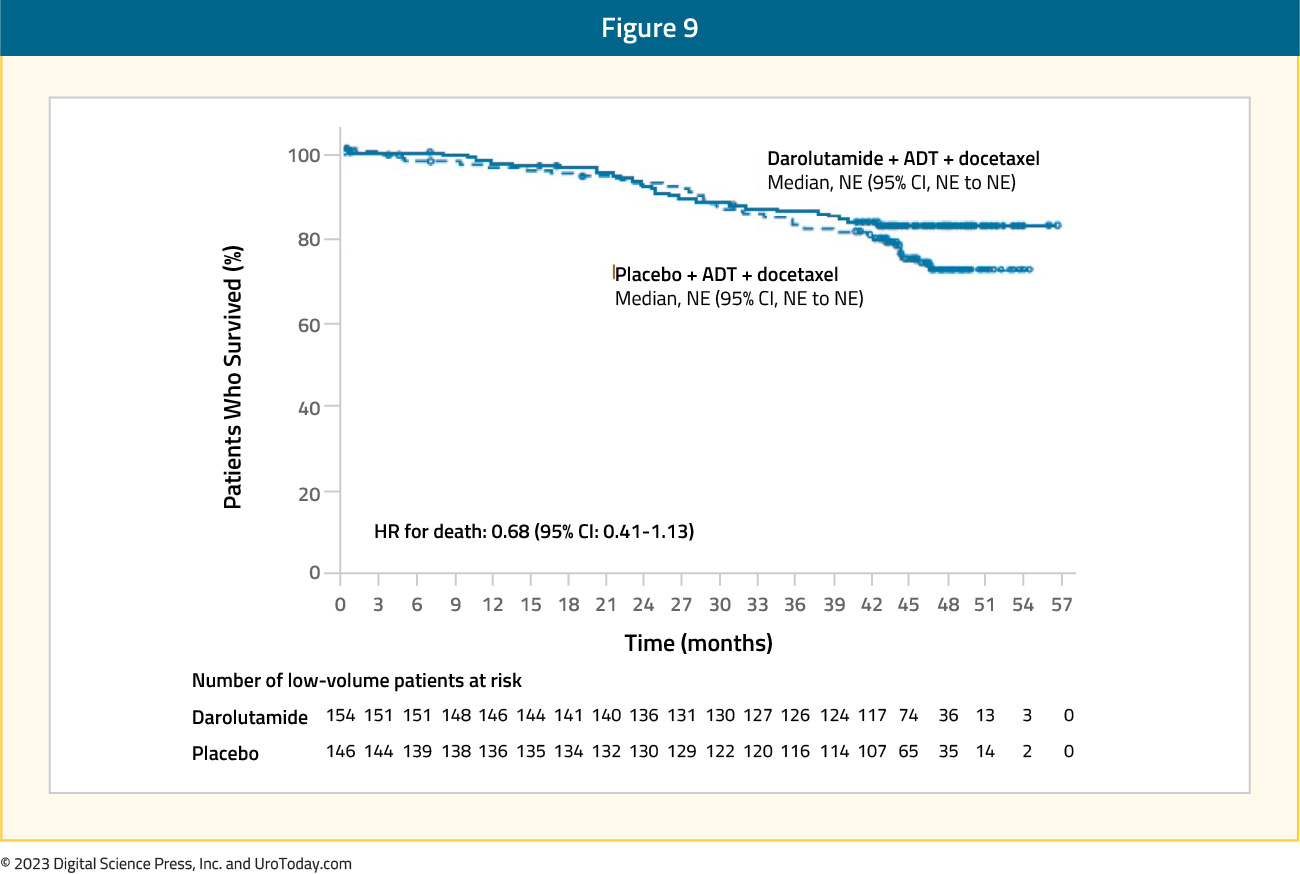
For patients with low volume disease, there appears to be a potential late clinical benefit to triplet therapy of ADT + docetaxel + darolutamide, however, with few events and additional follow-up time likely required, there is no statistically significant benefit at this point (HR: 0.68, 95% CI: 0.41 to 1.13)
Figure 9: Overall survival of darolutamide + ADT + docetaxel vs ADT + docetaxel for low volume disease patients in ARASENS
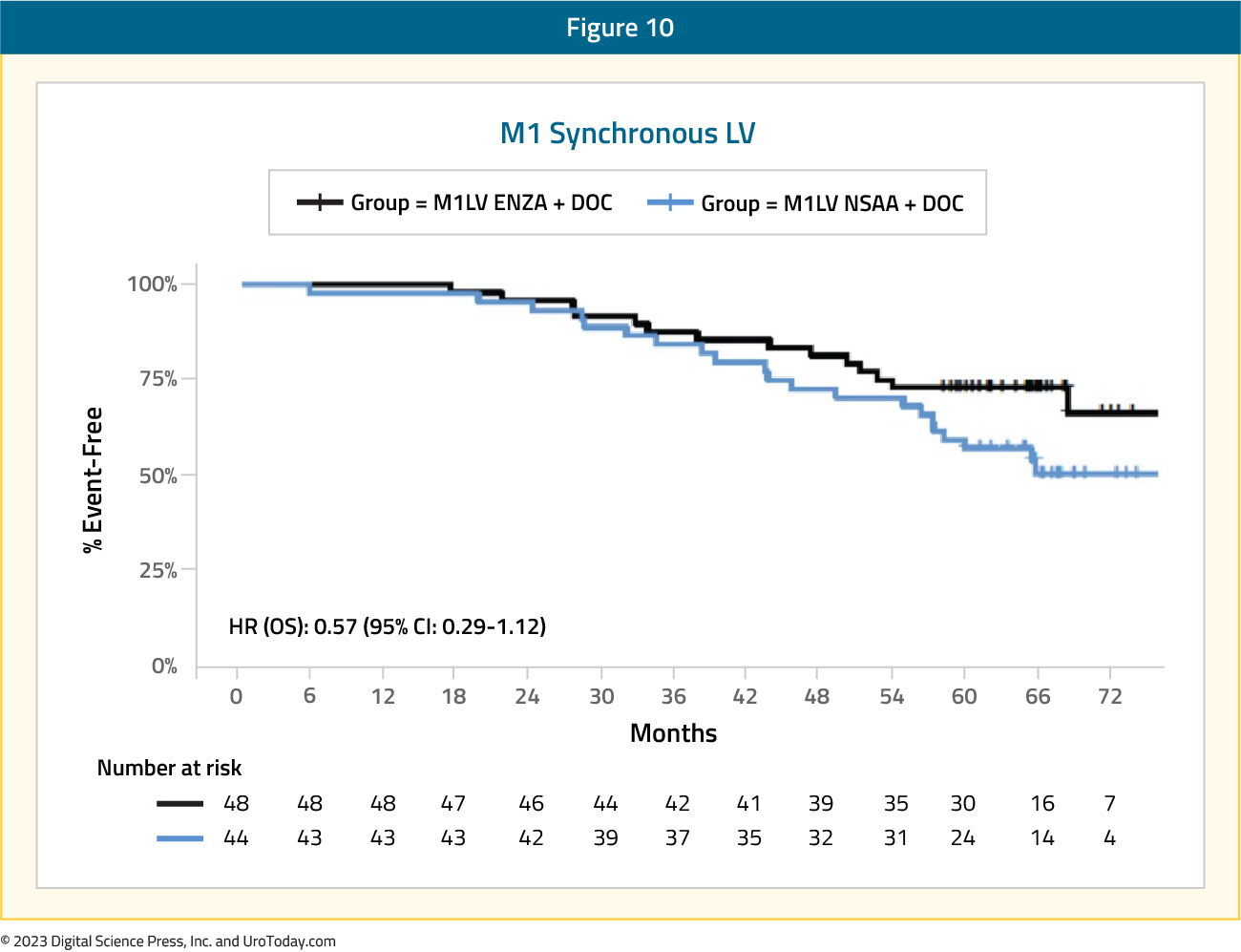
Similarly, for patients treated in the ENZAMET trial with low volume synchronous disease, there was no benefit for ADT + enzalutamide versus ADT + standard nonsteroidal antiandrogen for those planned for docetaxel (HR: 0.57, 95% CI: 0.29 to 1.12).
Figure 10: Overall survival of enzalutamide + ADT + docetaxel vs ADT + docetaxel + non-steroidal anti-androgen for synchronous low volume disease patients
ARATs + ADT
There is consistent evidence across all major published phase III trials to support an overall survival benefit to the addition of an ARPI to ADT in patients with synchronous low-volume disease. This is reflected, as follows:
- LATITUDE (abiraterone + ADT versus ADT alone; all de novo): HR 0.72, 95% CI 0.47 to 1.109
- STAMPEDE (abiraterone + ADT versus ADT alone; >90% de novo): HR 0.64, (95% CI 0.42 to 0.96)10
- TITAN (apalutamide + ADT versus ADT alone; 10% prior docetaxel): HR 0.52, 95% CI 0.35 to 0.7911
- ENZAMET (enzalutamide + ADT versus non-steroidal antiandrogen + ADT): HR 0.58, 95% CI 0.32 to 1.04 4
- ARCHES (enzalutamide + ADT versus ADT alone; 18% prior docetaxel): HR 0.66, 95% CI 0.43 to 1.0312
As such, ARPI addition to ADT has become the backbone of any treatment approach in patients with synchronous, low volume prostate cancer.
Primary Radiotherapy for synchronous, low volume mHSPC patients
Beyond systemic treatment intensification, local prostate-directed therapy may allow for local treatment intensification. While a surgical approach using radical prostatectomy has been described, high quality data are limited to radiotherapy. Of note, the SWOG 1802 trial is accruing patients with a surgical arm in the setting of mHSPC to further assess the impact of cytoreductive prostatectomy in this disease space. Three trials to date have evaluated the role of local radiotherapy in the prostate in patients with mHSPC.
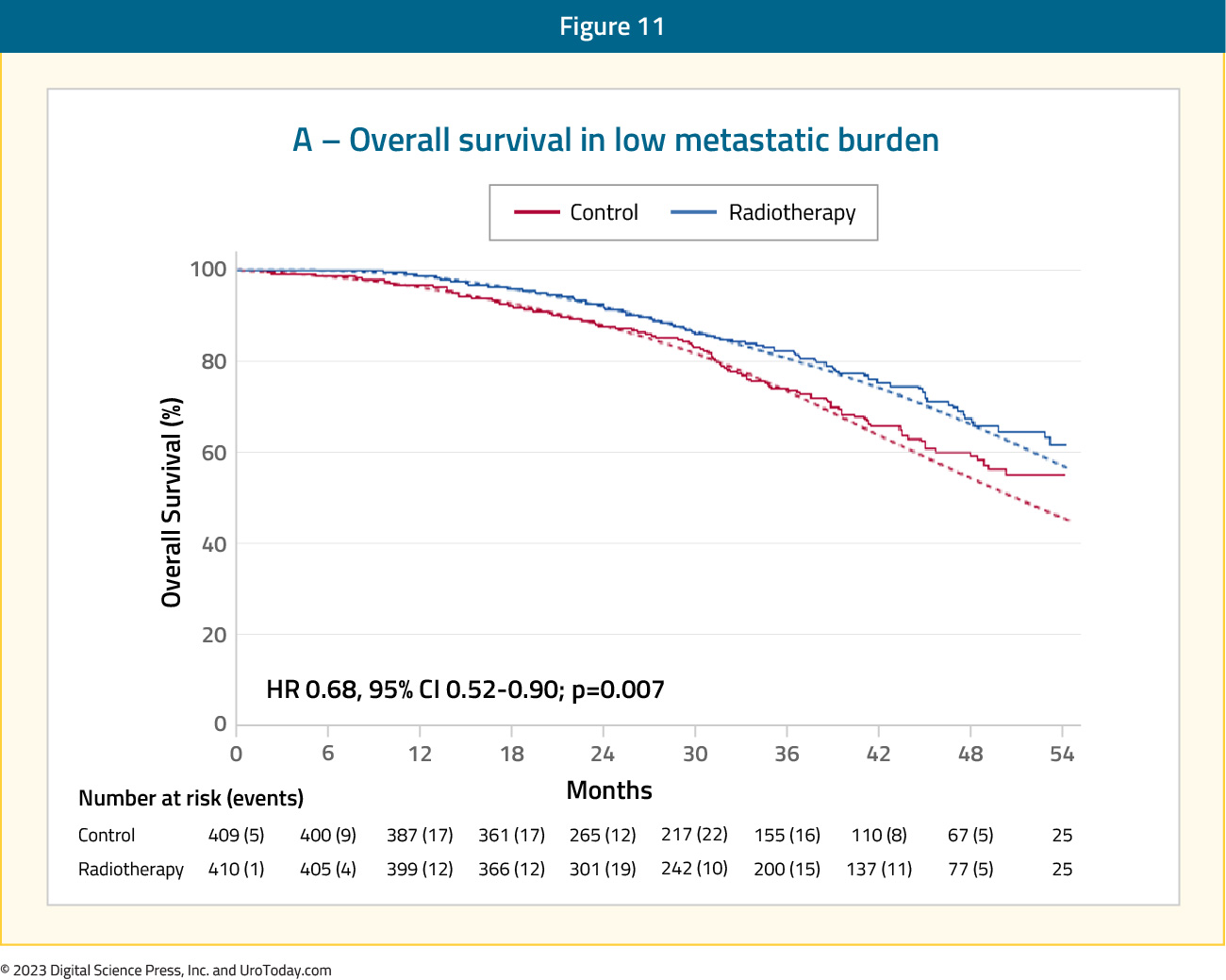
STAMPEDE (Arm H) was an open label, randomized controlled phase III trial of 2,061 men at 117 hospitals across Switzerland and the UK.11 This trial randomized patients with de novo mHSPC in a 1:1 fashion to standard of care + radiotherapy or standard of care alone. Men allocated to radiotherapy received either a daily (55 Gy in 20 fractions over 4 weeks) or weekly (36 Gy in six fractions over 6 weeks) schedule that was nominated before randomization. The primary outcome of this trial was overall survival. Subgroup analysis by metastatic volume (CHAARTED criteria) was planned a priori. Median follow up for STAMPEDE Arm H was 37 months, median patient age was 68 years, and median PSA was 97 ng/ml. 18% of patients received early docetaxel. In the overall cohort, radiotherapy improved failure-free survival (HR: 0.76, 95% CI:0.68 to 0.84) but not overall survival (HR: 0.92, 95% CI: 0.80 to 1.06). However, when stratified by metastatic burden, overall survival benefits were seen in the low volume group (HR: 0.68, 95% CI: 0.52 to 0.90) with restricted mean survival time improved by 3.6 months from 45.4 to 49.1.11
Figure 11: Overall survival in low metastatic burden patients with mHSPC and radiotherapy to the prostate primary in STAMPEDE Arm H
HORRAD was a multicenter prospective randomized clinical trial of 432 patients with previously untreated, de novo mHSPC at 28 centers across The Netherlands between November 2004 and September 2014. 20 All eligible patients had a PSA >20 ng/ml and documented bone metastases on bone scan. Patients were randomized in a 1:1 fashion to either ADT with EBRT or ADT alone, with a primary endpoint of overall survival. The median PSA was 142 ng/mL and over a median follow up of 47 months, the median overall survival was non-significantly different at 45 months in the radiotherapy + ADT arm compared to 43 months in ADT alone arm (HR: 0.90, 95% CI: 0.70 to 1.14).
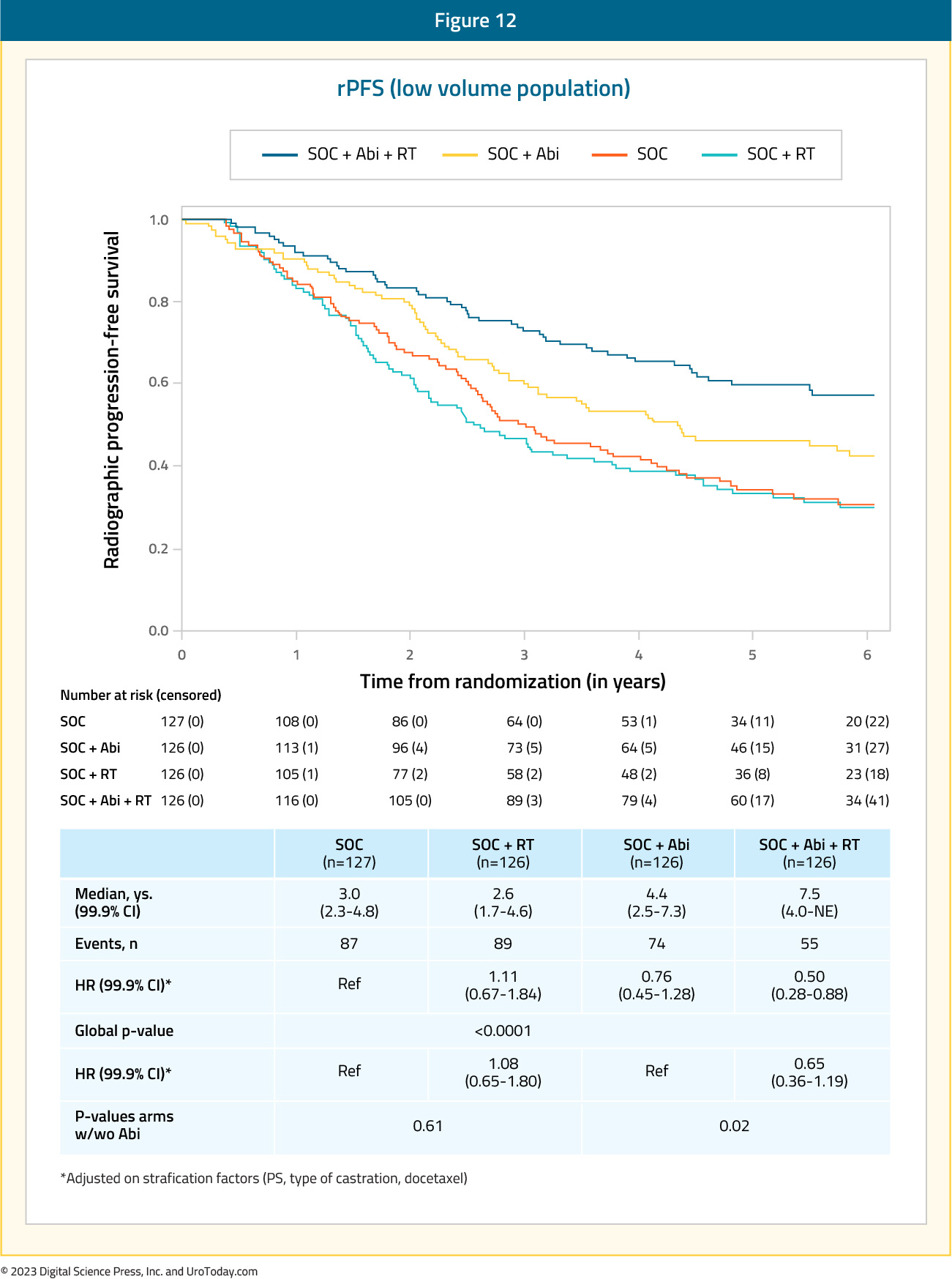
Results of the efficacy and safety of prostate radiotherapy for patients with low volume, de novo mHSPC from the PEACE-1 trial were recently presented at ASCO 2023. The addition of prostate radiotherapy to standard of care + abiraterone was associated with significant rPFS benefits (median 7.5 versus 4.4 years, p=0.02). Conversely, addition of radiotherapy to standard of care therapy alone was not associated with rPFS benefits (median 2.6 versus 3.0 years; HR: 1.11, 95% CI: 0.67 to 1.84, p=0.61).
The addition of prostate radiotherapy to either standard of care alone or standard of care therapy + abiraterone was not associated with overall survival improvements. In the standard of care + abiraterone arms, addition of prostate radiotherapy was associated with modest, non-significant OS benefits (HR: 0.77, 95% CI: 0.51 to 1.16, p=0.21). Similarly, addition of prostate radiotherapy to standard of care alone did not improve overall survival (HR: 1.18, 95% CI: 0.81 to 1.71, p=0.39).

Interestingly, addition of prostate radiotherapy to standard of care +/- abiraterone in the low-volume cohort was associated with significant improvements in the time to serious genitourinary events (p=0.0006). This overall benefit was consistent irrespective of whether patients had prostate radiotherapy added to standard of care + abiraterone (p=0.003) or standard of care therapy alone (p=0.048).
The majority of patients with synchronous, low volume mHSPC benefit from early systemic treatment intensification with ARPI addition to ADT. Such patients should also be offered primary radiotherapy to the prostate gland in the appropriate clinical settings.
Metachronous High Volume mHSPC
Docetaxel + ARPI + ADT
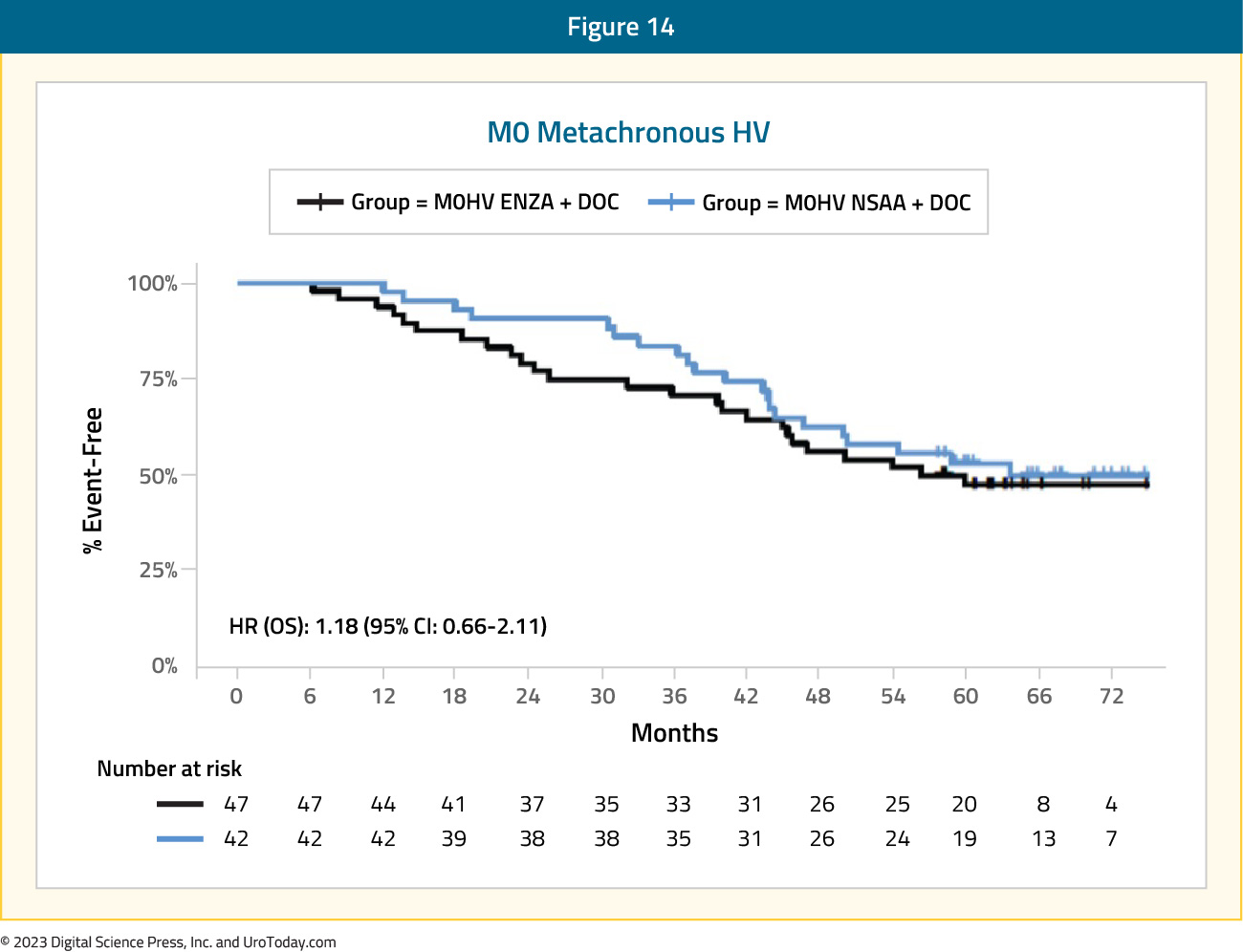
Given that the PEACE-1 trial included patients with de novo mHSPC only, ARASENS and ENZAMET provide the available data to assess the benefit of triplet therapy in this mHSPC subgroup. In ARASENS, the overall survival for patients with high volume mHSPC had a prespecified subgroup analysis for assessing recurrent (metachronous) disease (n =117) with a clinical benefit, but no statistically significant benefit (HR: 0.70, 95% CI: 0.39 to 1.24). In the ENZAMET trial, there was no benefit to treatment intensification for triplet therapy (HR: 1.18, 95% CI: 0.66 to 2.11).
Figure 14: Overall survival of enzalutamide + ADT + docetaxel vs ADT + docetaxel + non-steroidal anti-androgen for metachronous high volume disease patients in ENZAMET
ARPI + ADT
When considering patients with mHSPC along a risk continuum, from good risk (metachronous low volume: 8-year median overall survival with ADT alone) to poor risk (synchronous high volume: 3-year median overall survival with ADT alone), patients with synchronous low volume and metachronous high volume (median overall survival: 4.5 years with ADT alone) mHSPC may both be considered as intermediate risk disease. As such, the clinical treatment approach for these two subgroups has seen significant overlap. ARPI + ADT have similarly served as the backbone for treatment of these patients. Patients with metachronous, high volume mHSPC have historically accounted for only a small proportion of patients in the published phase III trials, and as such, post-hoc analyses have been underpowered for evaluating this subgroup. Results from the ARCHES trial have demonstrated a 23% decreased hazard of overall mortality in this subgroup with addition of enzalutamide to ADT (HR: 0.77, 95% CI: 0.39 to 1.50).5 Similar results were found in the ENZAMET trial (HR: 0.73, 95% CI: 0.37 to 1.44).
Docetaxel + ADT
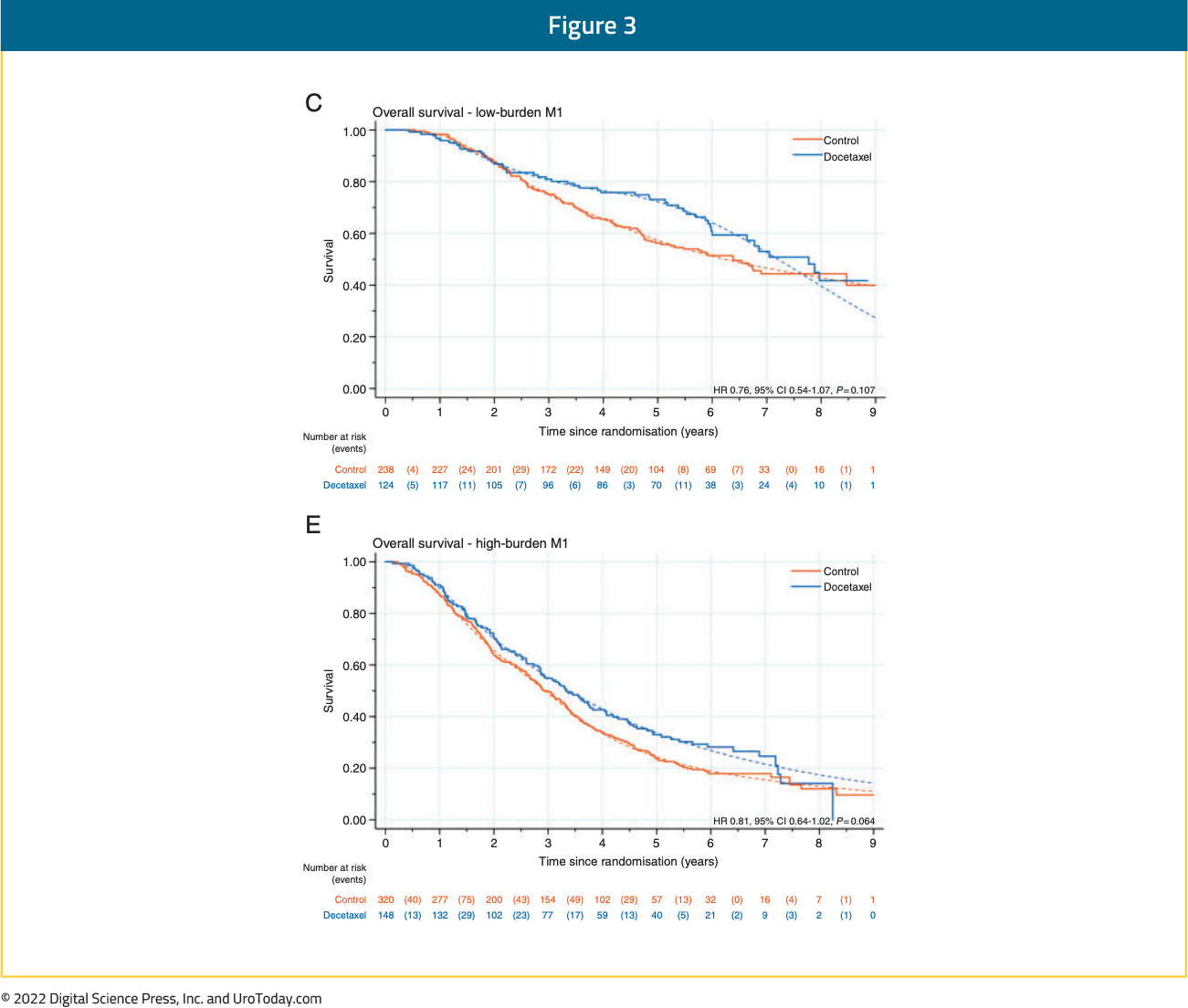
Results from the STOPCAP M1 collaborative meta-analysis of individual patient data from GETUG-15, STAMPEDE, and CHAARTED demonstrated that docetaxel addition to ADT in patients with metachronous, high volume prostate cancer is associated with significant improvements in overall survival (HR: 0.64, 95% CI: 0.42 to 0.99),21 which was consistent on follow-up analyses.22
Given the relatively increased toxicity with taxanes, along with a subset of patients being “chemotherapy unfit”, it appears that doublet therapy with an ARPI + ADT is the favored treatment approach in patients with metachronous high volume disease, with docetaxel reserved for select patients with higher volume of disease.
Metachronous Low Volume mHSPC
Docetaxel + ARPI + ADT
Similar to metachronous high volume patients, the subgroup analyses of triplet therapy trials assessing metachronous low volume mHSPC patients remain limited. In ARASENS, metachronous low volume disease patients (n = 51) had too few overall mortality events to provide adequate samples size for powered analyses. For the ENZAMET trial, the HR for metachronous low volume patients was 0.64 (95% CI: 0.18 to 2.28).
Figure 15: Overall survival of enzalutamide + ADT + docetaxel vs ADT + docetaxel + non-steroidal anti-androgen for metachronous low volume disease patients in ENZAMET

ARPI + ADT
Subgroup analyses have consistently demonstrated an overall survival benefit to ARPI addition in patients with low volume mHSPC. Results from the ARCHES trial demonstrated a 37% improved hazard of overall survival with enzalutamide addition to ADT in patients with metachronous low volume mHSPC (HR: 0.63, 95% CI: 0.26 to 1.54). From the ENZAMET trial, patients with metachronous low volume disease had a clinical and statistically significant benefit (HR of 0.47, 95% CI: 0.28 to 0.79).
Docetaxel + ADT
Importantly, there is consistent evidence against the use of docetaxel in this mHSPC subgroup. Results from the CHAARTED trial demonstrated a minimal overall survival in this subgroup (HR: 0.77, 95% CI: 0.51 to 1.18). Furthermore, results from the STOPCAP meta-analysis using CHAARTED and GETUG-AFU15 data demonstrated no overall survival benefit to docetaxel addition (HR: 1.07, 95% CI: 0.75 to 1.54).
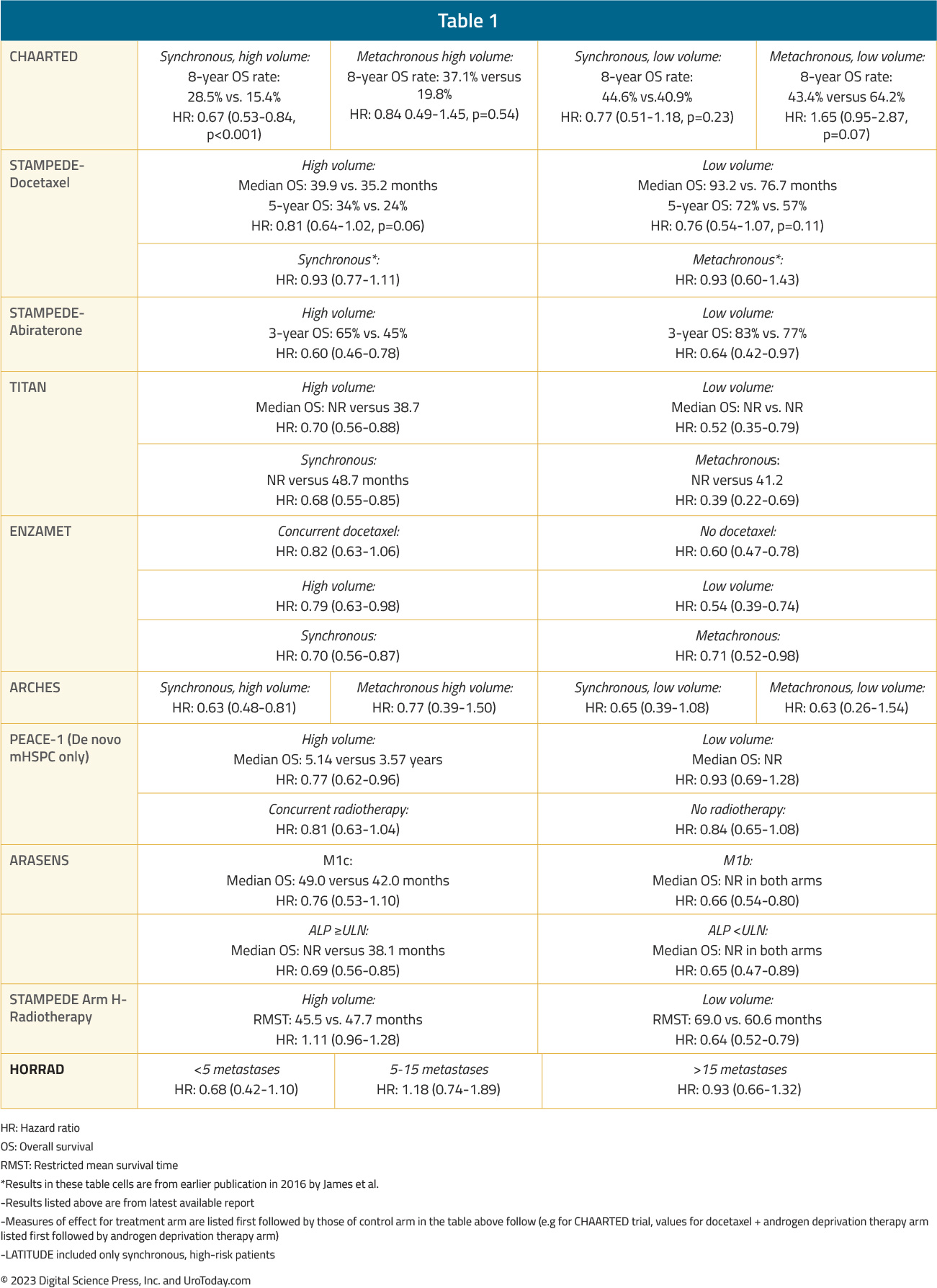
Table 1: A summary of overall survival by volume and timing of metastases from the key registration
Conclusions
Although there appears to be increasing utilization of treatment intensification in the real-world setting, less than half of mHSPC patients receive guideline concordant care. While there may be altruistic reasons to avoid treatment intensification secondary to concerns for patient financial toxicity or concerns for the tolerability of these agents, the proven survival benefit conferred by this treatment paradigm should make this approach the clear standard of care. Based on the current evidence, it appears that patients with synchronous, high volume mHSPC benefit from early treatment intensification with triplet therapy in the form of both an ARPI and docetaxel, whereas the remaining mHSPC subgroups benefit most from doublet therapy with ARPI addition to ADT. Radiotherapy to the prostate is also associated with improved overall survival in mHSPC patients with synchronous, low-volume disease and should be considered in these cases.
Related Content: New Pathways for Treating Metastatic Castration-Resistant Prostate Cancer (mCRPC)
Published November 2023
Part of an Independent Medical Education Initiative Supported by LOXO@Lilly