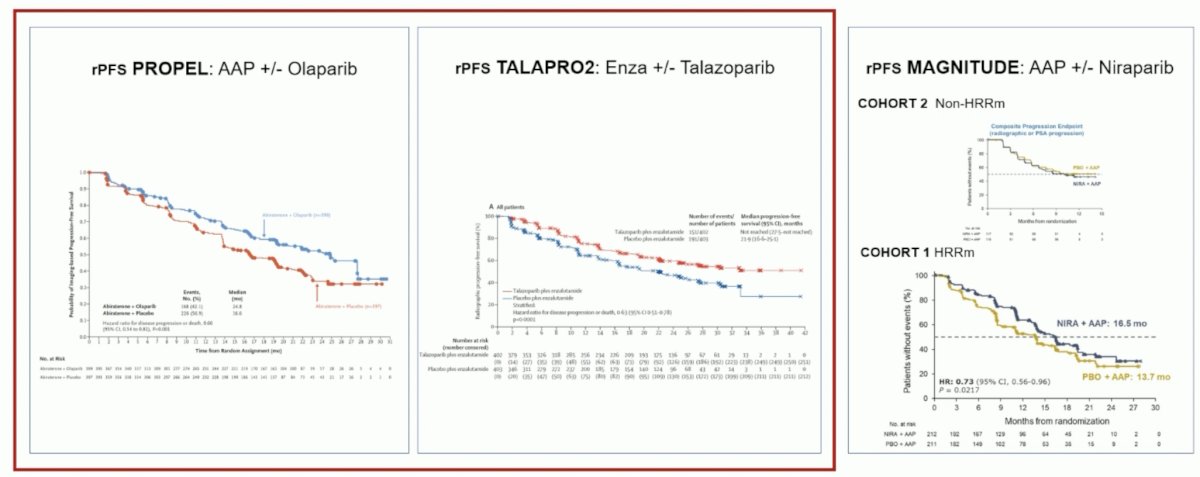
(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a plenary session on personalized approaches in high-risk and metastatic prostate cancer, and a presentation by Dr. Elena Castro discussing that PARP inhibitors should only be used for patients with alterations in DNA repair genes. Dr. Castro started by emphasizing that ARPI + PARP inhibitor trials in first-line mCRPC have shown rPFS benefits in patients unselected for HRR alterations in PROPEL1 and TALPARO-2,2 but not in MAGNITUDE3:
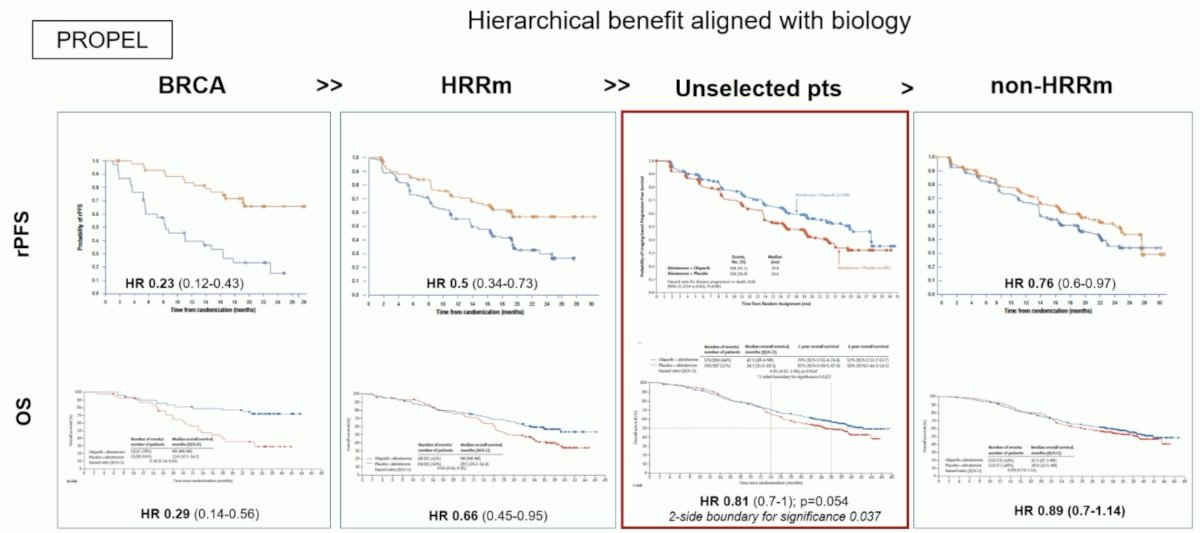
Notably, in PROPEL, there is a hierarchical benefit aligned with biology, whereby for both rPFS and OS, the best outcomes are seen in patients with BRCA mutations, followed by patients with HRR mutations, and the least benefit for patients with non-HRR mutations. Unselected patients fall in between those with HRR mutations and non-HRR mutations:
Importantly, there are different approvals by regulatory authorities:
- Olaparib + abiraterone acetate: EMA (all patients), FDA (BRCA 1/2)
- Talazoparib + enzalutamide: EMA (all patients), FDA (HRR mutated)
- Niraparib + abiraterone acetate: EMA (BRCA1/2), FDA (BRCA 1/2)

Ultimately, the non-HRR mutation population that may benefit from PARP inhibitors needs to be better characterized: Is there undetected HRR deficiency? Are there other events that may sensitize to PARP inhibitors? Synergy? Dr. Castro notes that these caveats do not justify treating all patients, but rather a justification for more research.
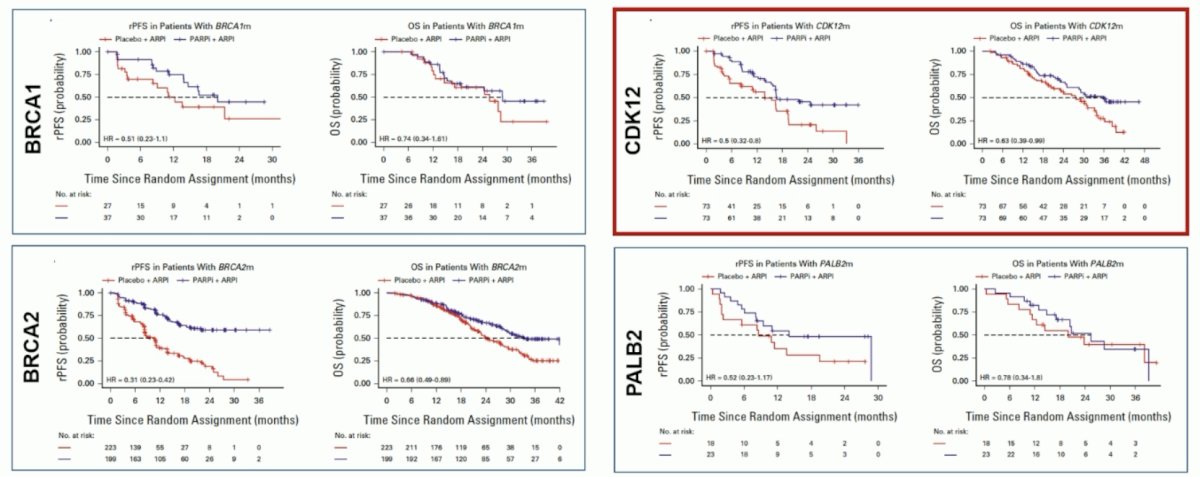
The HRR subgroup is heterogeneous, with different functions, prognostic implications, and sensitivity to PARP inhibition. In a recent FDA pooled gene analysis of MAGNITUDE, PROPEL, and TALAPRO-2,4 there was a benefit from ARPI + PARP inhibitors for patients with alterations in BRCA1, BRCA2, CDK12, and PALB2, but no apparent benefit for ATM and CHEK2:
Dr. Castro also notes that although there is increased toxicity with ARPI + PARP inhibitors versus ARPI alone, there is no difference in toxicity by HRR status:

Dr. Castro concluded her presentation by discussing that PARP inhibitors should only be used for patients with alterations in DNA repair genes with the following conclusions:
- How much benefit justifies the increased side effects and cost of intensified therapy?
- Increased cost and increased toxicity, versus
- Available biomarkers to identify those patients most likely to benefit
Presented by: Elena Castro, MD, Hospital Universitario, Madrid, Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024
References:
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1094-1108.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023 Jun 20;41(18):3339-3351.
- Fallah J, Xu J, Weinstock C, et al. Efficacy of Poly(ADP-ribose) Polymerase Inhibitors by Individual Genes in Homologous Recombination Repair Gene-Mutated Metastatic Castration-Resistant Prostate Cancer: A US Food and Drug Administration Pooled Analysis. J Clin Oncol. 2024 Mar [Epub ahead of print].
Related Content:
EAU 2024: PARP-Inhibitors Only for Patients with Alterations in DNA Repair Genes: No
EAU 2024: PARP-Inhibitors Only for Patients with Alterations in DNA Repair Genes: The Guidelines' View


