(UroToday.com) The 2024 ESMO annual meeting included a highlights session, featuring a presentation by Dr. Ignacio Duran discussing highlights of the ESMO 2024 non-prostate cancer sessions. Dr. Duran started by highlighting the outline of his presentation discussing the most relevant presentations in kidney cancer and bladder cancer:

Over the last couple of years, it has been relatively status quo in the mRCC disease space. So, can we add any additional benefit to our patients if we introduce immunotherapy at the time of progression? Is it useful to sequence immunotherapeutic agents in mRCC? For context, Dr. Duran briefly discussed the CONTACT-03, which randomized patients that have progressed on previous immune checkpoint inhibitor treatment to atezolizumab + cabozantinib versus cabozantinib alone.1 After a median follow-up of 15.2 months, the median progression-free survival was 10.6 months (95% CI 9.8-12.3) with atezolizumab + cabozantinib and 10.8 months (95% CI 10.0-12.5) with cabozantinib (HR 1.03, 95% CI 0.83-1.28):
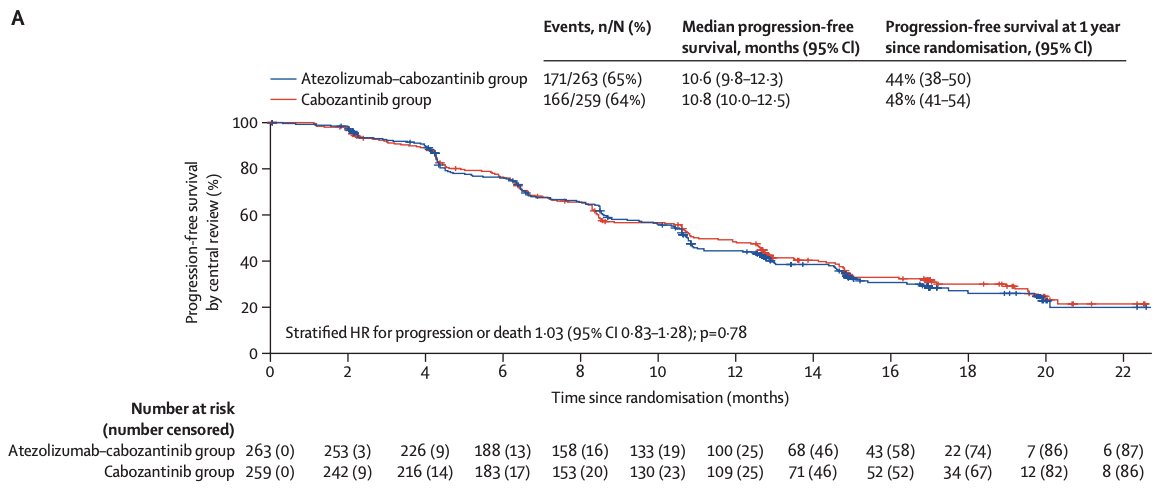
The median overall survival was 25.7 months (95% CI 21.5-not evaluable) with atezolizumab + cabozantinib and was not evaluable (21.1-not evaluable) with cabozantinib (HR 0.94, 95% CI 0.70-1.27):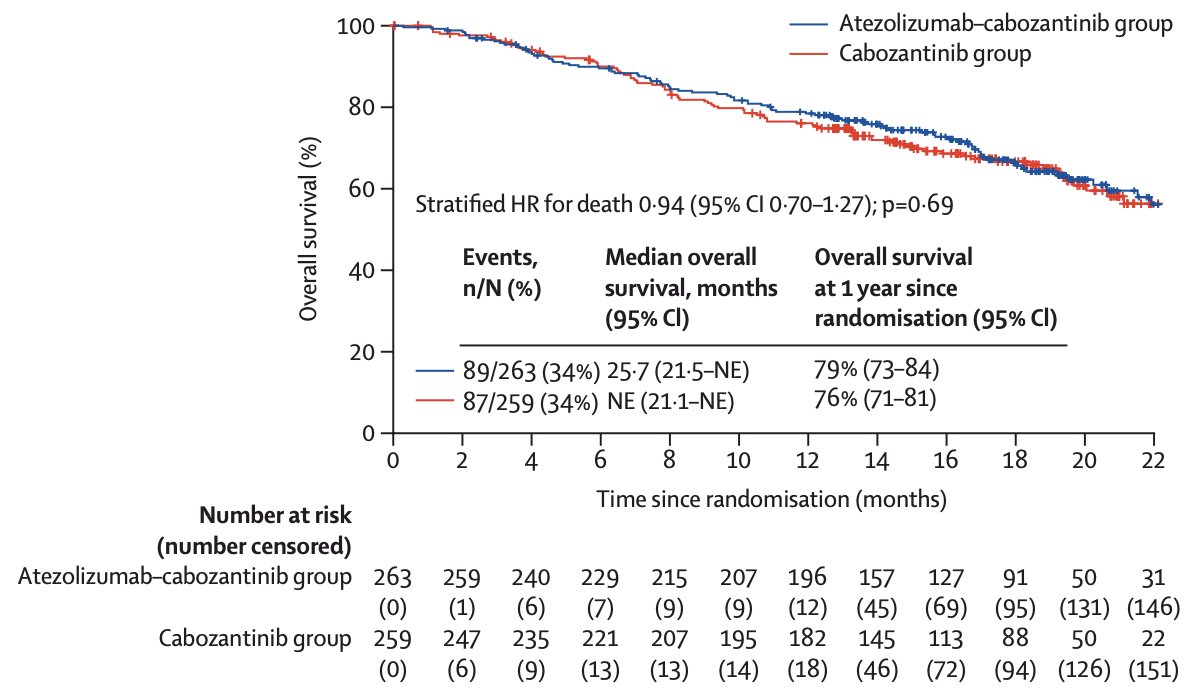
With this background, Dr. Duran discussed “Tivozanib–Nivolumab vs Tivozanib Monotherapy in Patients with RCC Following 1 or 2 Prior Therapies including an Immune Checkpoint Inhibitor – Results of the Phase III TiNivo-2 Study.” He notes there are subtle differences between CONTACT-03 and TiNivo-2, including re-challenging with a different checkpoint inhibitor along with a different TKI.
TiNivo-2 enrolled patients with advanced clear-cell RCC who had 1–2 prior lines of therapy, including an immune checkpoint inhibitor. Patients were randomized to receive tivozanib (0.89 mg) plus nivolumab or tivozanib alone (1.34 mg). The primary endpoint was progression-free survival by independent radiology review. The key secondary endpoint was overall survival, and other secondary endpoints included investigator-assessed progression-free survival, objective response rate, duration of response, and safety/tolerability. The trial design for TiNivo-2 was as follows: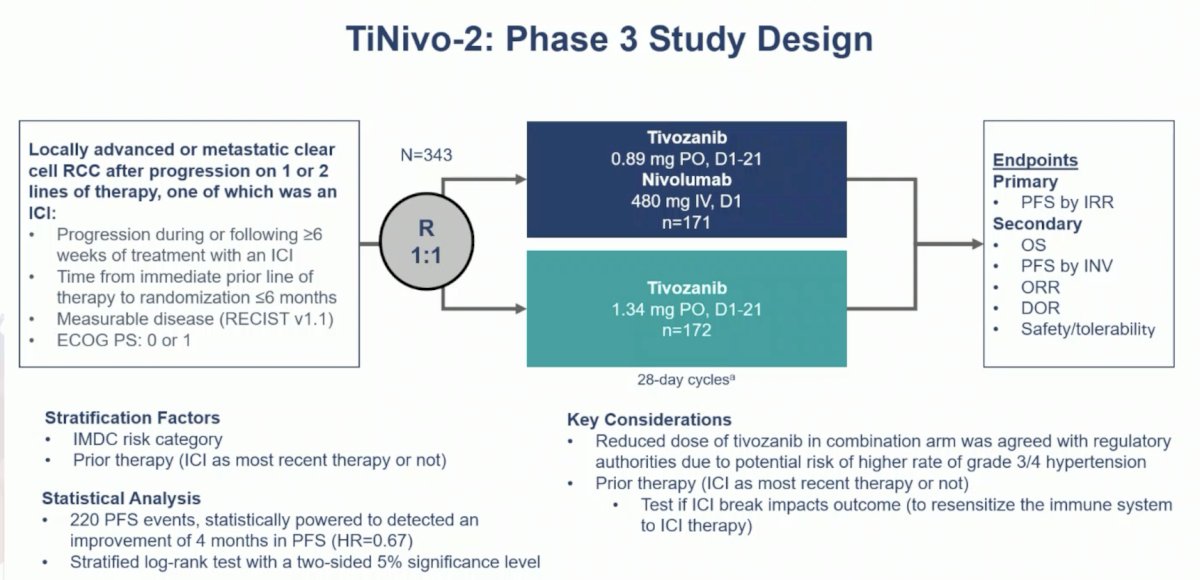
There were 343 patients randomized to tivozanib + nivolumab (n = 171) or tivozanib (n = 172), with well balanced baseline characteristics:
With a median independent radiology review-assessed progression-free survival of 5.7 months for tivozanib + nivolumab and 7.4 months for tivozanib (HR 1.10, 95% CI 0.82-1.43), the study did not meet its primary endpoint: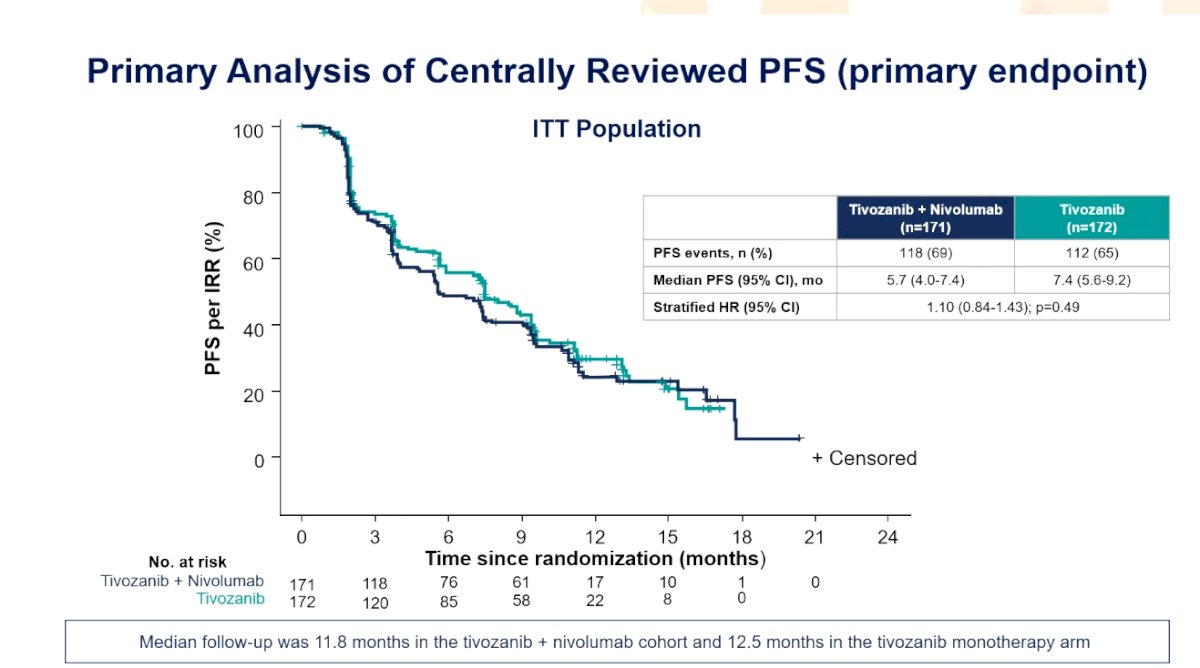
Further, there was no progression-free survival benefit when stratified by immune checkpoint inhibitors at the most recent therapy, non-immune checkpoint inhibitors at the most recent therapy, second-line therapy, or third-line therapy. The median overall survival for tivozanib + nivolumab was 17.7 months (95% CI 15.1 – not reached) and for tivozanib monotherapy was 22.1 months (95% CI 15.2 – not reached; HR 1.00, 95% CI 0.68 – 1.46):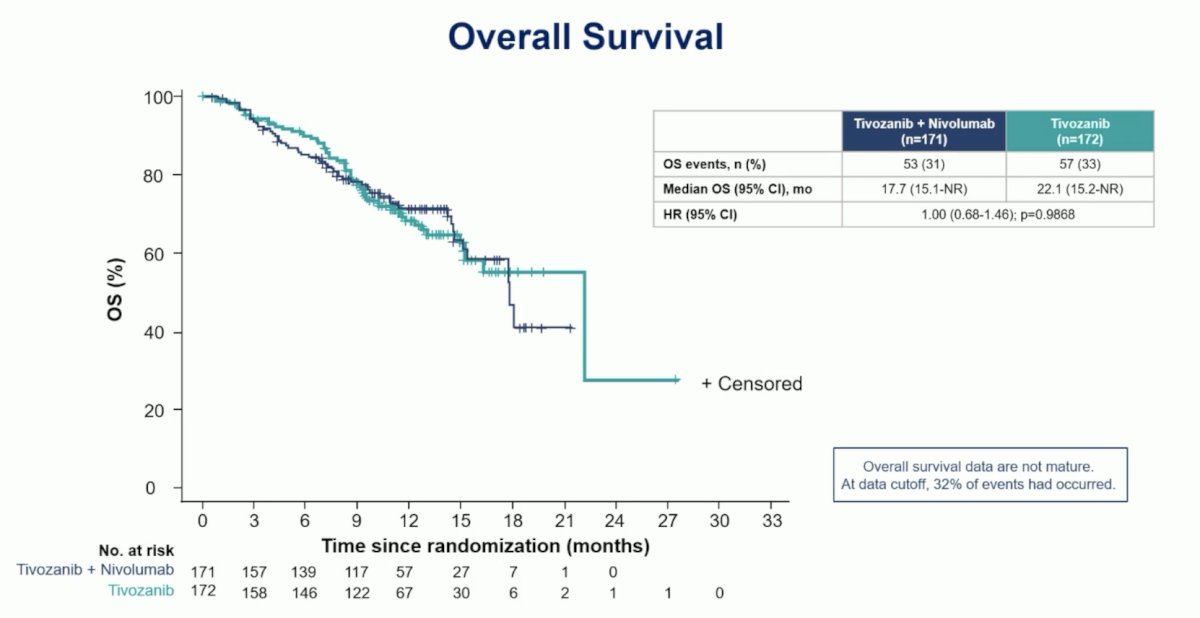
Dr. Duran’s take-home messages from TiNivo-2 are as follows:
- We have enough evidence to fully recommend against sequencing checkpoint inhibitors in mRCC in our daily practice
- Two phase III randomized trials (including over 800 patients) have demonstrated that the use of checkpoint inhibitors upon progression to previous checkpoint inhibitors (received either in the immediate previous line or in further back lines of therapy) does not add any benefit and increases toxicity.
- TKI as a single agent remains the standard of care upon progression on checkpoint inhibitor therapy based on treatment in mRCC and tivozanib now represents a valid alternative to cabozantinib
Switching to non-clear cell RCC, Dr. Duran notes that there are limited high-quality studies to guide the management of a non-clear cell histologies (in particular the non-papillary histologies). Despite variable activity reported with different compounds (single agent or combinations), no drug or combination has been able to demonstrate a solid improvement in overall survival. Dr. Duran then discussed “Prospective randomized phase II trial of Ipilimumab + Nivolumab versus standard of care in non-clear cell RCC: Results of the SUNNIFORECAST trial”. This trial randomly assigned patients with non-clear cell RCC in a 1:1 ratio to receive either nivolumab 3 mg/kg IV combined with ipilimumab 1 mg/kg IV every 3 weeks for 4 doses followed by a flat dose of 240 mg IV every 2 weeks or 480 mg every 4 weeks versus standard of care by investigators choice until disease progression or intolerance occurred. Patients were stratified in papillary versus non-papillary non-clear cell RCC and according to the IMDC risk score. The trial design for SUNNIFORECAST is as follows:
A total of 316 patients were screened in 7 European countries and 31 centers. The randomized population of 309 patients (70.9% male, 29.1% female) were randomized to receive either nivolumab plus ipilimumab (157 patients) or standard of care (152 patients: 124 TKI, 17 TKI/IO, 2 other treatment regimens, 9 patients did not receive any treatment):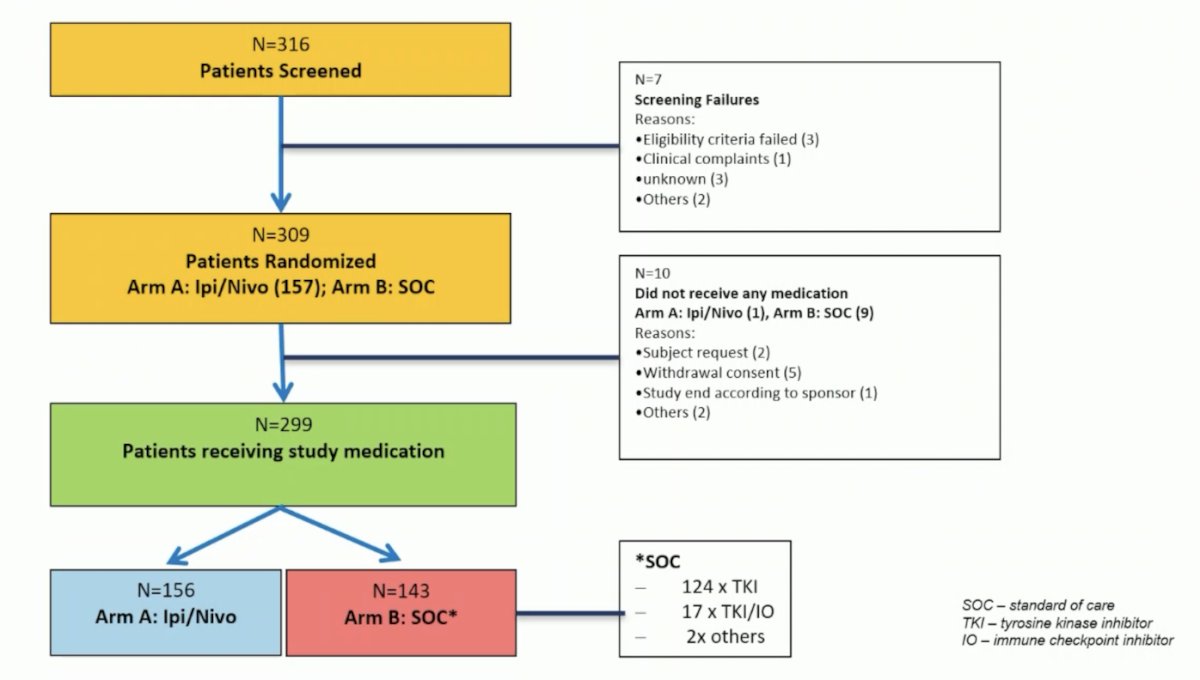
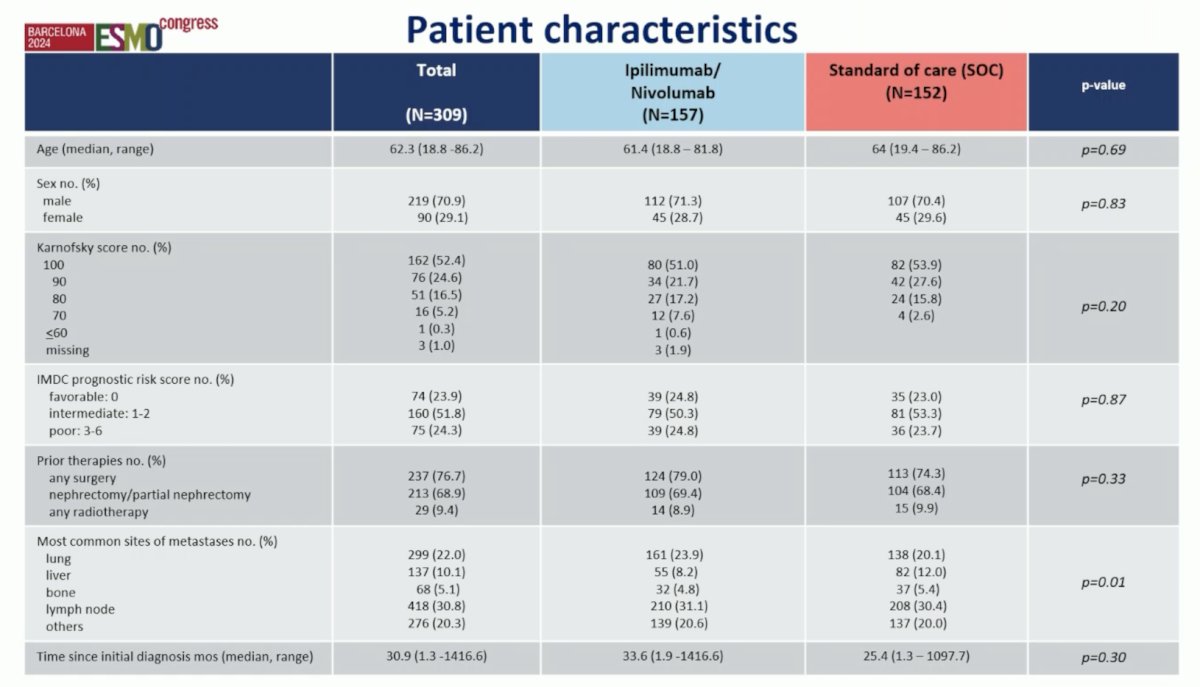
There were 178 patients (57.6%) with papillary, 60 patients (19.4%) with chromophobe, 12 patients (3.9%) with MIT, 9 patients (2.9%) with collecting duct carcinoma, and 50 patients with other subtypes. According to the IMDC score, 23.9% were of low, 51.8% of intermediate, and 24.3% of high risk. The 12-month overall survival rate for the entire population was 82.5%. The 12-month overall survival rate for Ipilimumab + Nivolumab 86.9% (95%-CI 80.2%-91.5%) was statistically significantly superior to the standard of care 76.8% (95%-CI 68.6%-83.1%) (p = 0.014). Median overall survival was 42.4 months for the Ipilimumab + Nivolumab arm and 33.9 months for the standard-of-care arm: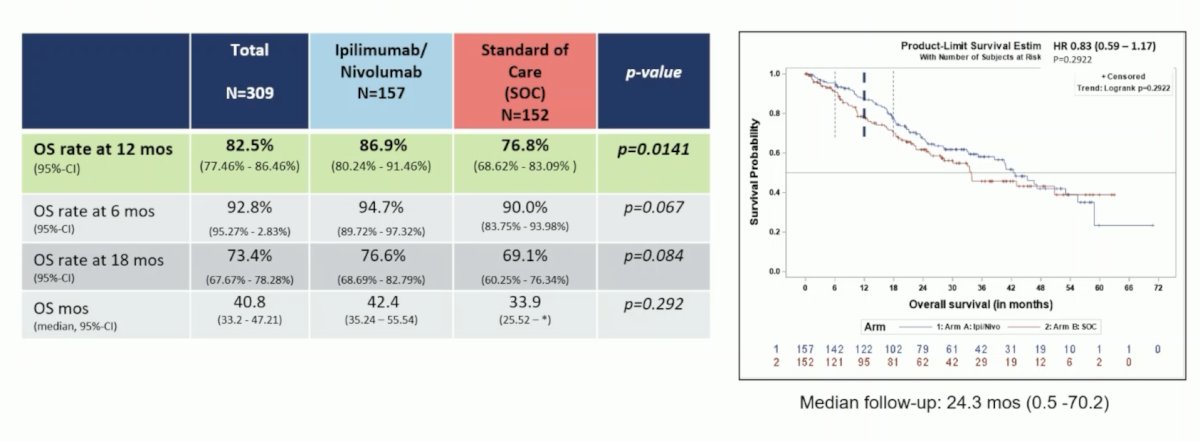
Dr. Duran noted that there are some questions around the potential effects of the underuse of cabozantinib, the lack of sustained benefit in the long term, and some lack of efficacy data. His summary points from SUNNIFORECAST are as follows:
- This is the first prospective randomized trial with checkpoint inhibitors in nonclear cell RCC
- The overall survival rate at 12 months was significantly superior for ipilimumab + nivolumab versus standard of care (predominantly TKI monotherapy) in nonclear cell RCC, thus the primary endpoint was met
- The overall survival and objective response rate suggests a benefit for ipilimumab + nivolumab, although some methodological aspects need to be solved
Other relevant RCC studies presented at ESMO acknowledged by Dr. Duran include:
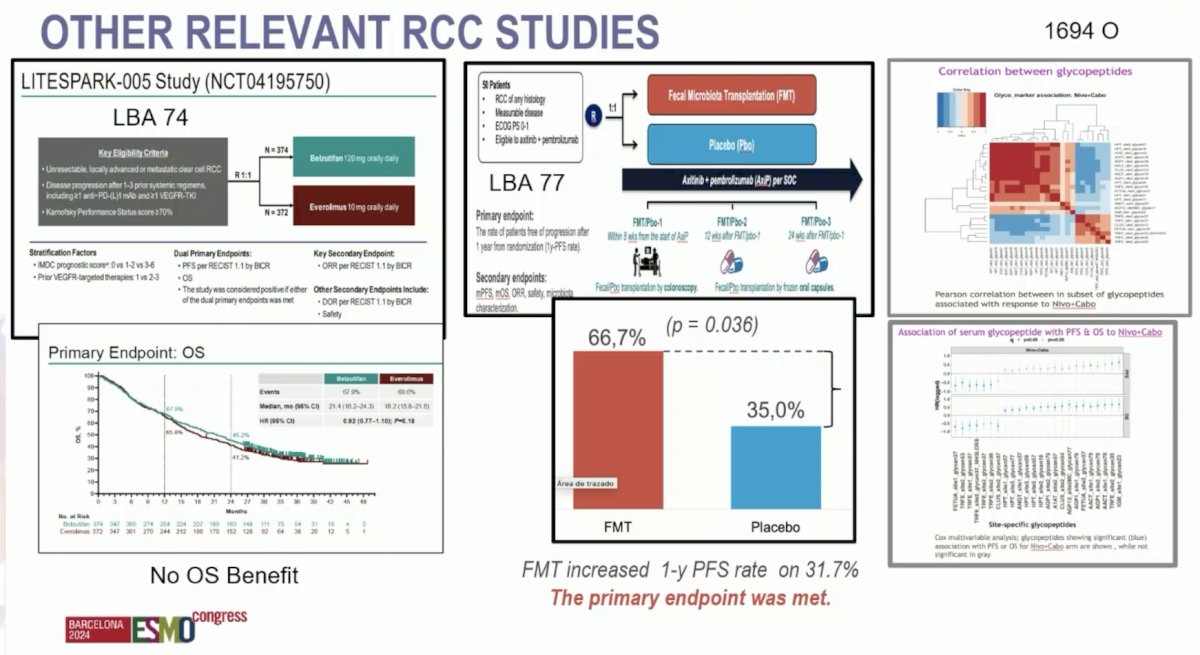
- Final analysis of the phase 3 LITESPARK-005 study of belzutifan versus everolimus in participants with previously treated advanced clear cell RCC
- Fecal microbiota transplantation versus placebo in patients receiving pembrolizumab plus axitinib for mRCC: Preliminary results of the randomized phase 2 TACITO trial
- Novel serum glycoproteomic biomarkers predict response to nivolumab plus cabozantinib versus sunitinib in advanced RCC: Analysis from CheckMate 9ER.
Dr. Duran concluded this portion of his presentation discussing highlights of ESMO 2024 in kidney cancer with the following take-home messages:
- We have enough evidence to recommend against sequencing of checkpoint inhibitors in mRCC
- TKIs as a single agent remain the standard of care upon progression to checkpoint inhibition in mRCC
- Based on the results of SUNNIFORECAST, ipilimumab + nivolumab could be considered as another valid option along with IO-TKI in advanced nonclear cell mRCC.
- Belzutifan is superior in progression-free survival versus everolimus but failed to improve overall survival in advanced clear cell mRCC in heavily pretreated patients.
- The fecal microbiota transplantation could be an interesting strategy in mRCC and future studies may help define next approaches.
- The search for biomarkers continues in RCC (ie. serum glycopeptides)
Switching to key abstracts presented at ESMO 2024 in bladder cancer, Dr. Duran notes that the current standard approach to muscle-invasive bladder cancer is neoadjuvant chemotherapy followed by radical cystectomy followed by adjuvant immunotherapy for high-risk patients, and surveillance for low-risk patients: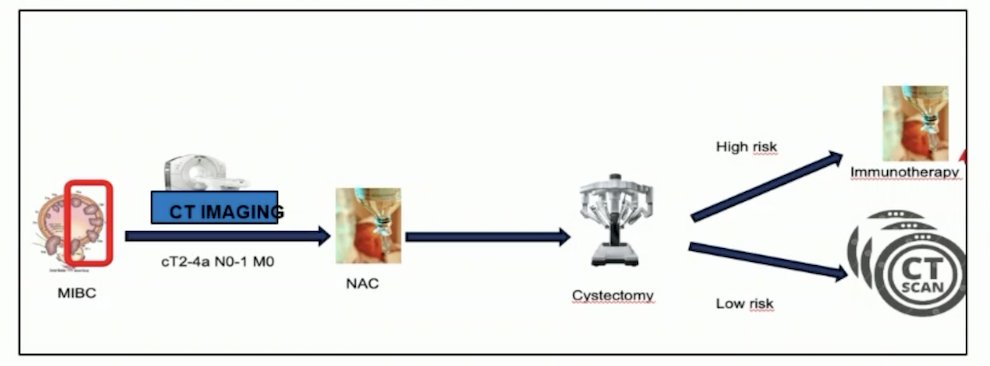
At ESMO 2024, three studies were presented that may challenge this paradigm for muscle-invasive bladder cancer. The first trial discussed was “TAR-200 plus cetrelimab or cetrelimab alone as neoadjuvant therapy in patients with muscle-invasive bladder cancer who are ineligible for or refuse neoadjuvant cisplatin-based chemotherapy: Interim analysis of SunRISe-4.” Patients underwent 5:3 randomization to:
- TAR-200 225 mg gemcitabine every 3 weeks (indwelling) for 12 weeks + cetrelimab intravenously for 12 weeks (4 cycles)
- Cetrelimab intravenously for 12 weeks (4 cycles)
Randomization was stratified by the absence or presence of visible residual disease at TURBT and tumor stage at muscle-invasive bladder cancer diagnosis (cT2 versus cT3-4a). The planned sample size was n = 160, and the primary endpoint was a pathologic complete response. Key secondary endpoints were recurrence-free survival, safety, pathologic objective response (i.e., ≤ypT1N0), and overall survival. For this interim analysis, the clinical data cutoff was May 31, 2024.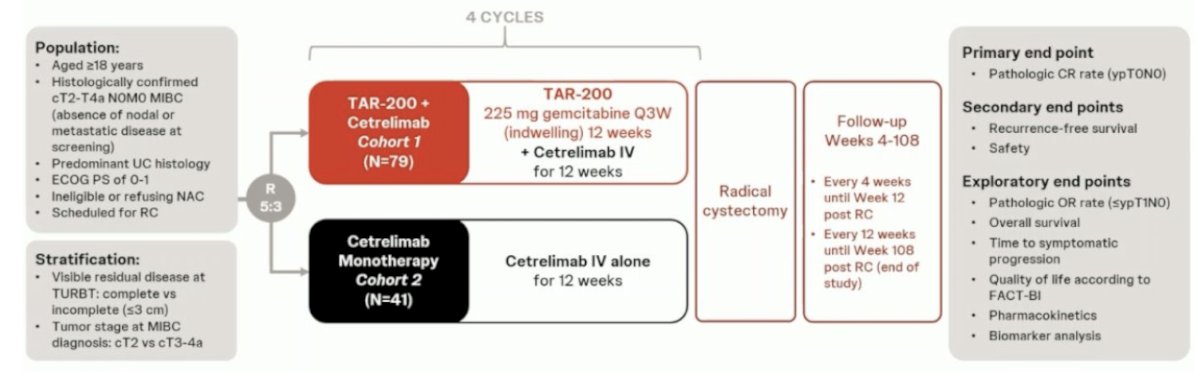
The pathologic complete response rate in the combination arm was 42%, and the pathologic objective response (pathologic complete response rate + ≤ypT1N0) was 60%. The corresponding proportions in the cetrelimab monotherapy arm were 23% and 36%, respectively:
Next, these outcomes were assessed, stratified by clinical stage, and completeness of TURBT. In cT2 patients, the pathologic complete response rate and pathologic objective response rates were 48% and 68% respectively. Conversely, in cT3-4a patients, the corresponding rates were lower at 23% and 39%, respectively. Patients with an incomplete TURBT (n = 9 only) had pathologic complete response rate and pathologic objective response rates of 56% and 67%, respectively. Those with a complete TURBT had rates of 39% and 59%, respectively: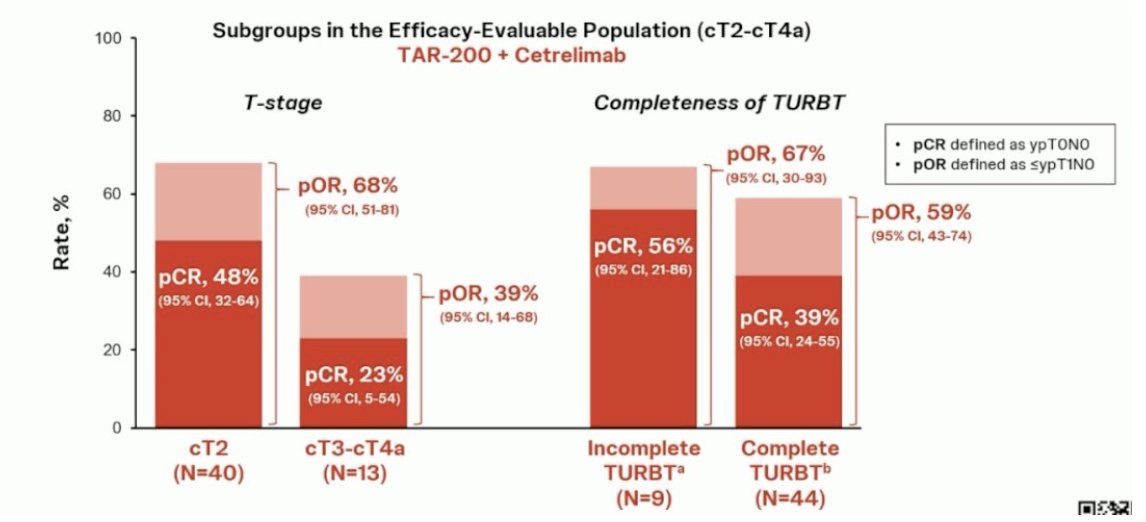
The efficacy of TAR-200 improved with an increasing number of doses administered. The pathologic complete response rate proportion increased from 27% to 30% to 50% in patients who received 1–2, 3, and the full 4 doses, respectively:
Dr. Duran summarized the SunRISe-4 trial with the following points:
- The combination of neoadjuvant TAR-200 + cetrelimab showed pathologic complete response rate and pathologic objective response rates of 42% and 60%, respectively, in patients with muscle-invasive bladder cancer
- In the cT2 subgroup, 48% of patients treated with TAR-200 + cetrelimab achieved pathologic complete response, and 68% were downstaged to ≤pT1 at radical cystectomy.
- Cetrelimab monotherapy provided pathologic complete response rate and pathologic objective response rates of 23% and 35%, respectively.
- TAR-200 + cetrelimab had a manageable safety profile in the neoadjuvant setting
- Most treatment-related adverse events with TAR-200 + cetrelimab were low-grade.
- The rate of discontinuations due to treatment-related adverse events was low at 13%
- SunRISe-4 demonstrates for the first time a benefit of the addition of TAR-200, an intravesical targeted releasing system, to checkpoint inhibition as neoadjuvant treatment in patients with muscle-invasive bladder cancer.
The second trial in this disease space discussed was “Identification of bladder cancer patients that could benefit from early post-cystectomy immunotherapy based on serial circulating tumour DNA (ctDNA) testing: preliminary results from the TOMBOLA trial.” TOMBOLA was a national, non-randomized ctDNA-based intervention study conducted at 5 centers in Denmark. Eligible patients were those with cT2-4aN0-1M0, cisplatin, and immunotherapy-eligible MIBC who underwent neoadjuvant chemotherapy followed by radical cystectomy. Patients underwent serial ctDNA testing post-operatively. Upon ctDNA detection, patients were recommended for one year of atezolizumab therapy: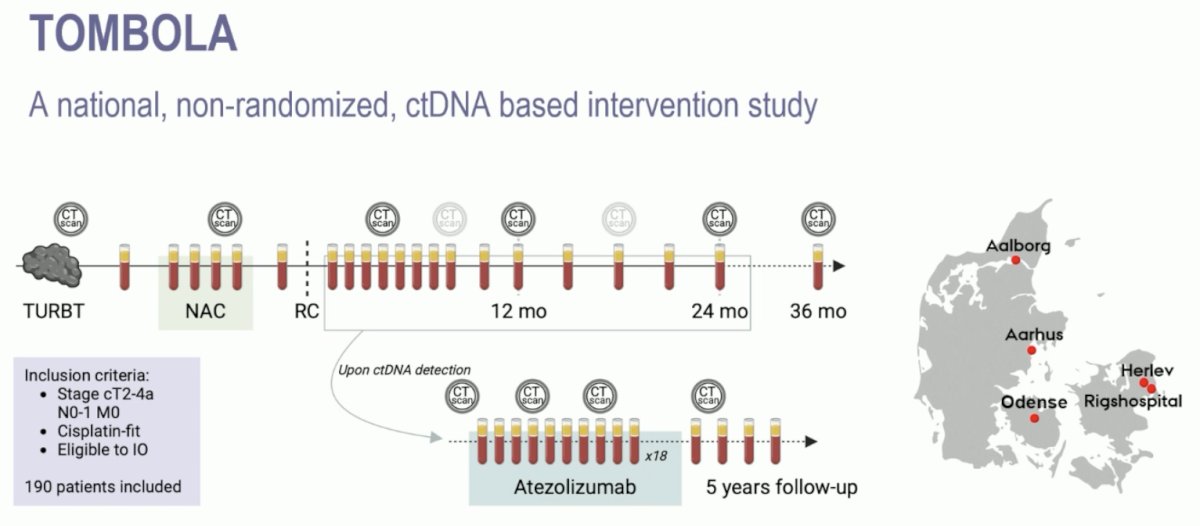
There were 56% of patients ctDNA positive post-radical cystectomy, and 75% were detected <4 months post-cystectomy. Of the ctDNA-negative patients, only 2 (3%) developed metastases on CT scan during follow-up.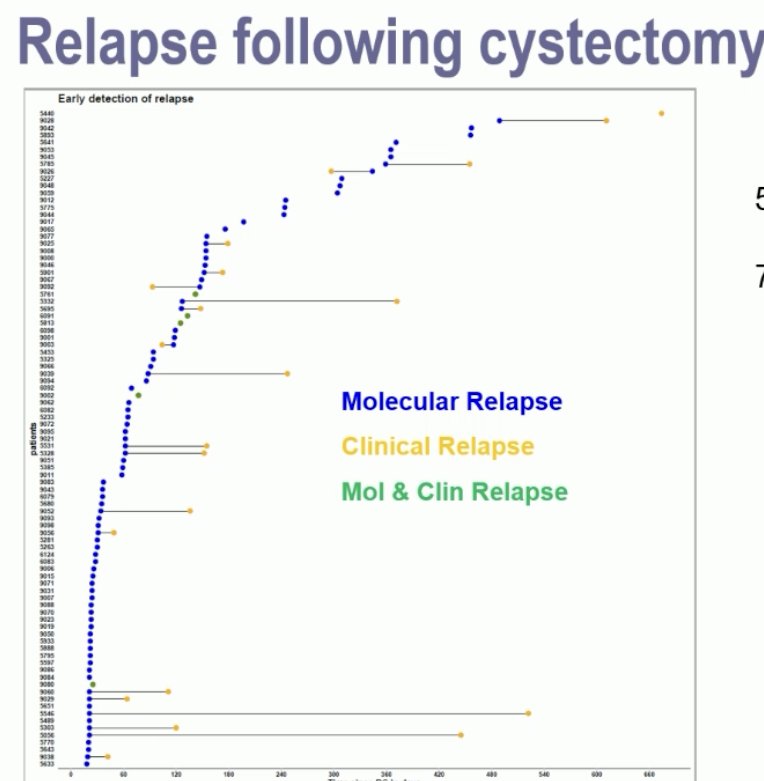
In the ctDNA-negative patients, both recurrence-free and overall survivals were excellent, as demonstrated in the Kaplan-Meier curves below: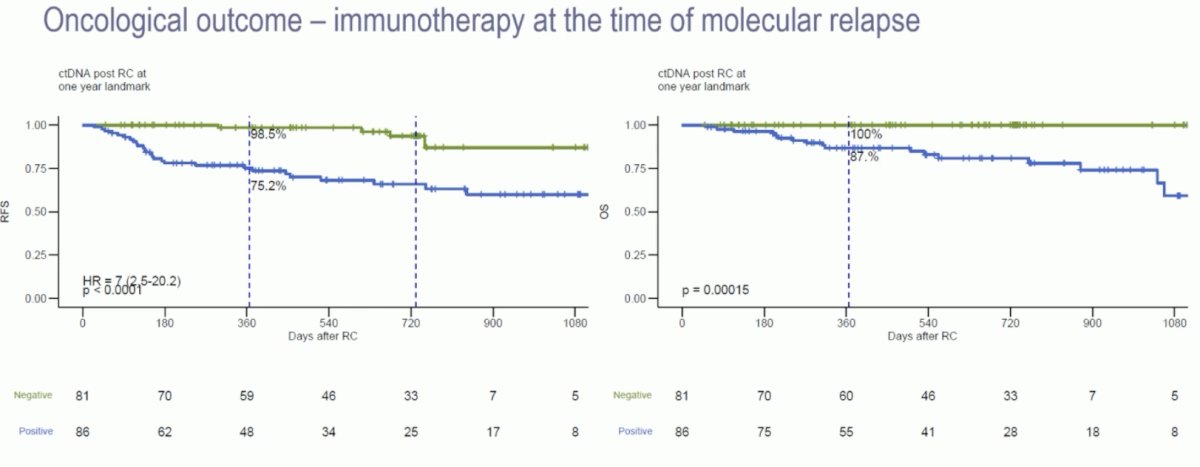
Thus, Dr. Duran notes that serial measurements of ctDNA followed by neoadjuvant chemotherapy and radical cystectomy is a highly specific method to identify patients that may benefit from early immunotherapy at a time of minimal metastatic disease.
The third trial in this disease space is the NIAGARA phase 3, randomized, open-label, multicenter trial that enrolled cisplatin-eligible patients with muscle-invasive bladder cancer (cT2-T4aN0/1M0) planned for radical cystectomy Patients were randomized (1:1) to receive either neoadjuvant durvalumab (1500 mg IV Q3W) and neoadjuvant chemotherapy (cisplatin + gemcitabine IV Q3W) for 4 cycles followed by radical cystectomy then adjuvant durvalumab monotherapy (1500 mg IV Q4W) for 8 cycles (durvalumab arm), or neoadjuvant chemotherapy followed by RC alone (comparator arm). The trial design for NIAGARA is as follows: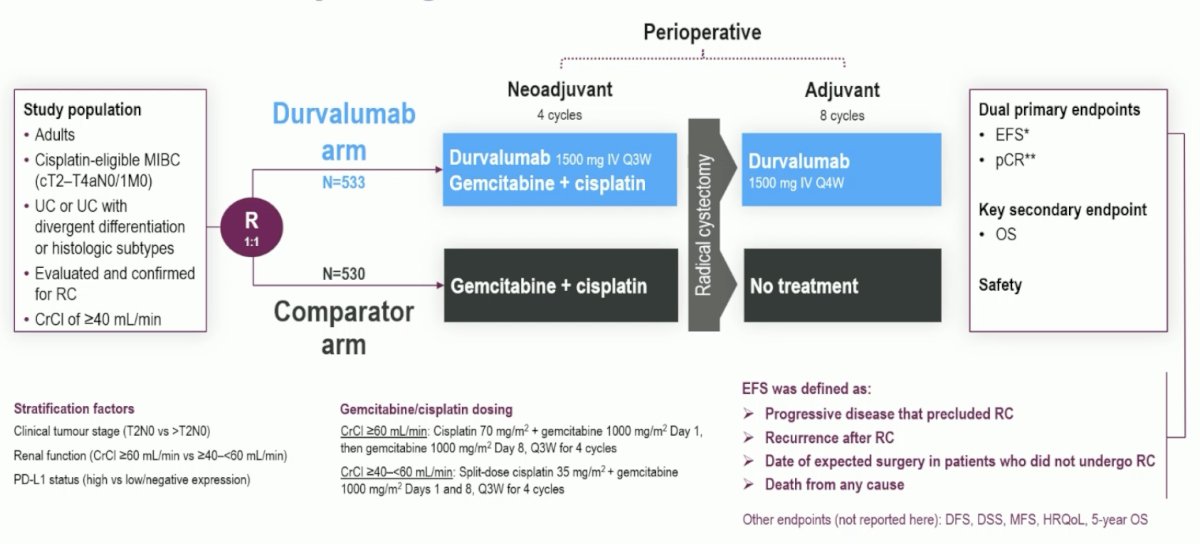
At the time of the formal analysis (January 2022), 33.8% of patients in the durvalumab arm had a pathologic complete response versus 25.8% in the comparator arm (OR 1.49, 95% CI 1.14-1.96). At the time of re-analysis (April 2024), the pathologic complete response rate in the durvalumab arm was 37.3% compared to 27.5% in the comparator arm (OR 1.60, 95% CI 1.23-2.08):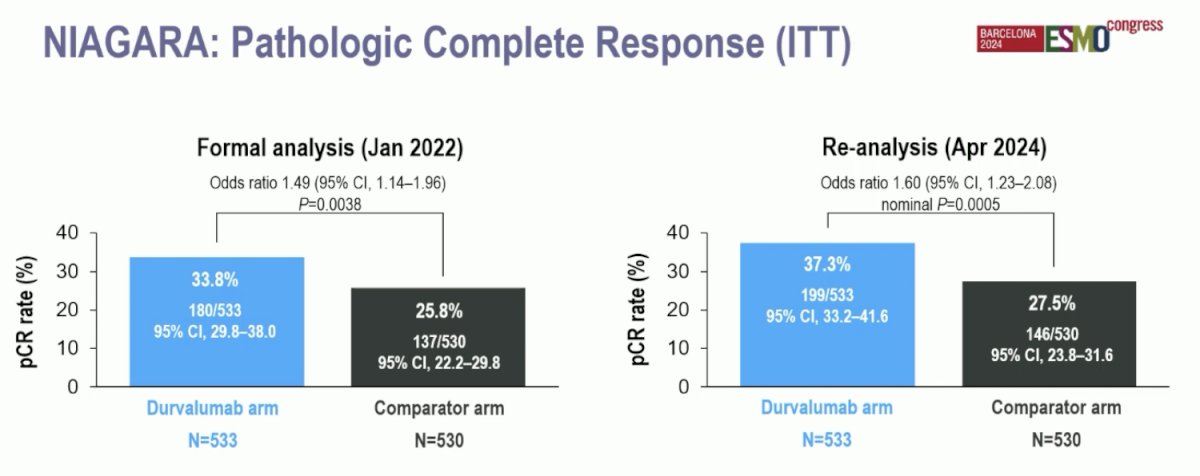
Event-free survival, as assessed by blinded independent central review in the intention-to-treat population, showed a significant reduction in the hazard of events in the Durvalumab arm. The event-free survival at 2 years was 67.8% in the Durvalumab arm compared to 59.8% in the comparator arm. The HR for event-free survival was 0.68 (95% CI: 0.56–0.82, p<0.0001):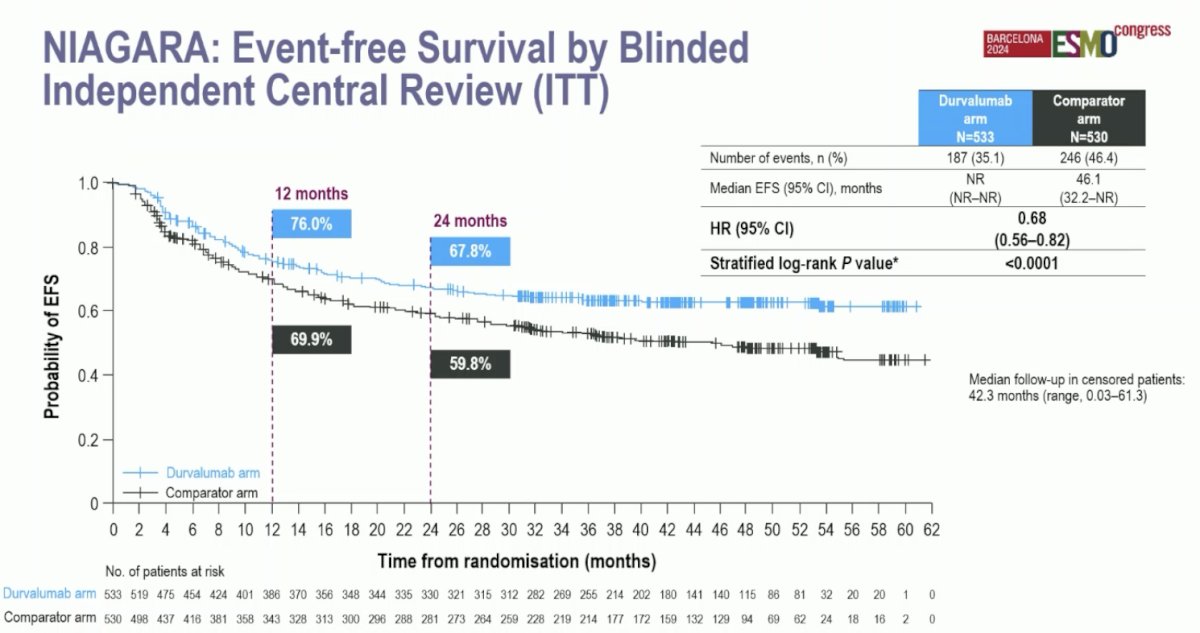
The median follow-up for the overall survival analysis in censored patients was 46.3 months (range 0.03–64.7). At 24 months, 82.2% of patients in the durvalumab arm were alive, compared to 75.2% in the comparator arm. The hazard ratio for overall survival was 0.75 (95% CI: 0.59–0.93, p = 0.016):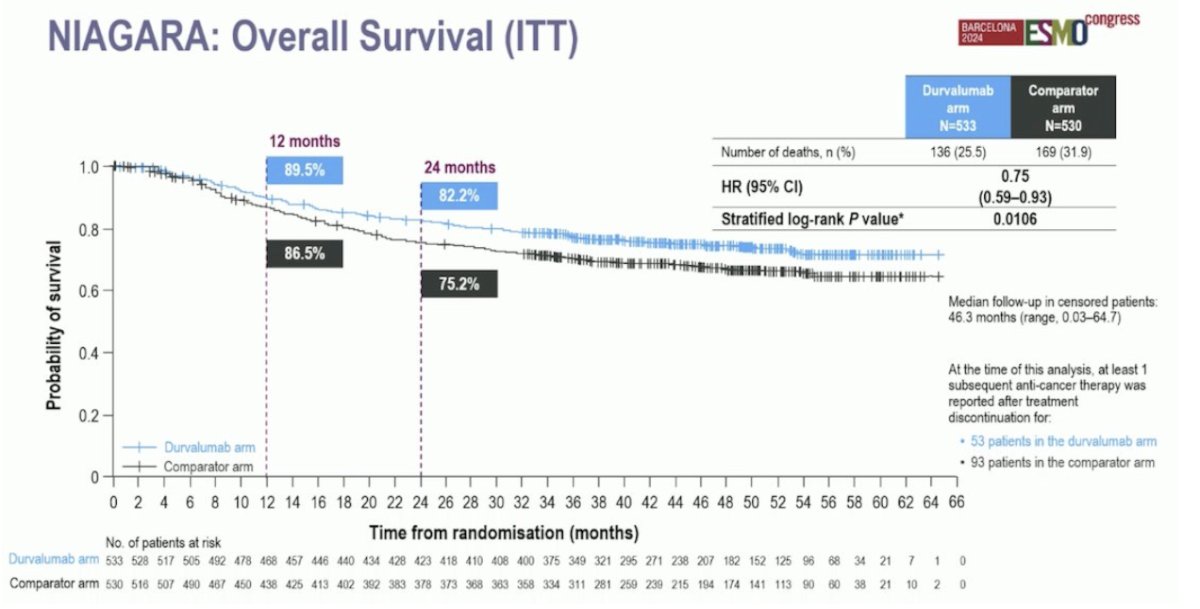
In terms of safety, adverse events were well-balanced between the two arms. Grade 3/4 events possibly related to treatment occurred in 41% of patients in both arms. Adverse events leading to patients not undergoing radical cystectomy occurred in 1% of patients in both groups. Adverse events delayed surgery in 2% of the durvalumab arm and 1% of the patients in the comparator arm. Discontinuation of neoadjuvant chemotherapy was 14% in the durvalumab arm and 15% in the comparator arm and death occurred in 5% of patients in the durvalumab group and 6% in the comparator arm:
Dr. Duran summarized NIAGARA with the following messages:
- Niagara is the first phase 3 perioperative immunotherapy study in muscle-invasive bladder cancer and has demonstrated a statistically significant and clinically meaningful improvement in event-free survival and overall survival
- The event-free survival and overall survival benefits with durvalumab were consistent across subgroups
- The pathologic complete response results and the significant overall survival benefit further support the perioperative approach
- The addition of perioperative durvalumab to neoadjuvant chemotherapy was tolerable, with no new safety signals. Most adverse events were manageable, and Grade 3/4 events were similar in both treatment arms.
- NIAGARA supports perioperative durvalumab with neoadjuvant chemotherapy as a potential new standard treatment for patients with cisplatin-eligible muscle-invasive bladder cancer
Dr. Duran briefly mentioned two abstracts in the metastatic urothelial carcinoma disease space that were impactful:
- Phase 2 study of futibatinib plus pembrolizumab in patients with advanced/metastatic urothelial carcinoma: Final analysis of efficacy and safety
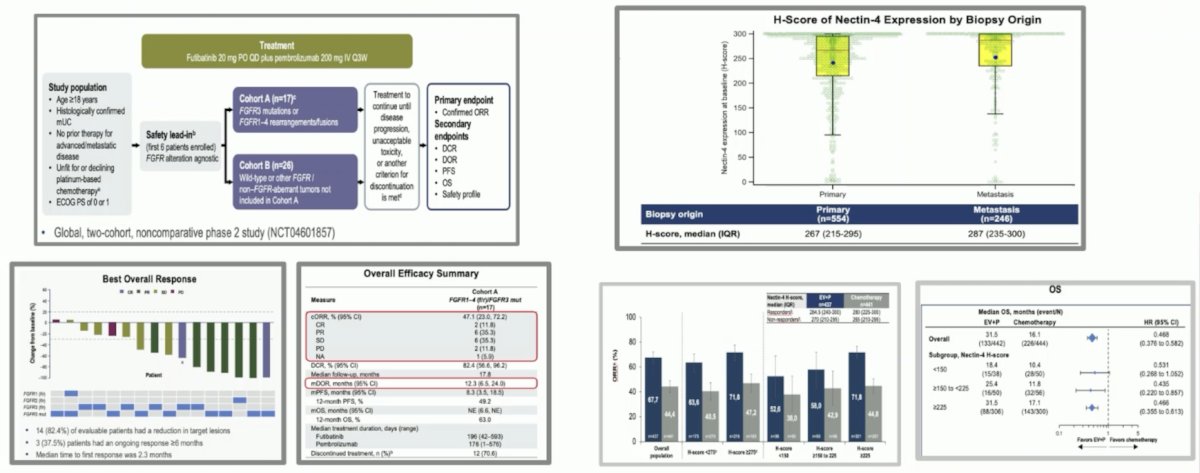
- EV-302: Exploratory analysis of nectin-4 expression and response to first-line enfortumab vedotin + pembrolizumab in previously untreated locally advanced or metastatic urothelial cancer:
Finally, the following abstracts were not specifically highlighted in the non-prostate GU tumors session highlights, but deserve mentioning:
Dr. Duran concluded this portion of his presentation discussing highlights of ESMO 2024 in kidney cancer with the following take-home messages:
- New approaches such as intravesical devices are being developed in muscle-invasive bladder cancer for nonplatinum eligible patients and strategies to refine preoperative treatment (ie. ctDNA) are more relevant
- The use of neoadjuvant chemotherapy + durvalumab followed by adjuvant durvalumab has demonstrated an improvement in event-free survival and overall survival when compared with chemotherapy alone and could represent a new standard of care in this setting.
- Nectin-4 expression does not appear to select patients for enfortumab vedotin
- FGFR remains a valid target in metastatic urothelial carcinoma and different combinations are being explored
Presented by: Ignacio Duran, MD, PhD, Hospital Universitario Marques de Valdecilla, Santander, Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Pal SK, Albiges L, Tomczak P, et al. Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): A multicenter, randomized, open-label, phase 3 trial. Lancet 2023 Jul 15;402(10397):185-195.
Highlights in Non-Prostate GU Oncology from ESMO 2024 - Ignacio Duran
TiNivo-2 Trial Results: Tivozanib in Advanced Renal Cell Carcinoma Treatment - Toni Choueiri
SunRISe-4 Trial Explores TAR-200 and Cetrelimab Combination for Bladder Cancer Treatment - Andrea Necchi


