Introduction
Poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors are drugs that prevent the repair of DNA single-stranded breaks and promote their conversion to double-stranded breaks resulting in a synthetic lethality.1 These drugs have demonstrated promising results for the treatment of metastatic castrate-resistant prostate cancer (mCRPC) patients who experience disease progression following prior androgen receptor pathway inhibitor (ARPI) and/or taxane-based chemotherapy.There is growing interest in combining these agents with other classes of drugs that may have synergistic mechanisms of action. A prime example of this is the use of combination PARP inhibitors and APRIs, with ARPIs inhibiting the transcription of specific homologous recombination repair (HRR) genes, inducing an HRR deficiency-like state, which potentiates PARP inhibitor activity, and, conversely, PARP inhibitors upregulating androgen receptor signaling, enhancing ARPI activity.2-4 This has culminated in the approval of three PARP inhibitor/ARPI combinations by the US Food and Drug Administration (FDA) for the treatment of mCRPC patients in the first line setting:
- Olaparib plus abiraterone for BRCA1/2-mutated patients5
- Niraparib plus abiraterone for BRCA1/2-mutated patients6
- Talazoparib plus enzalutamide for HRR-mutated patients7
PARP Inhibitors + Radium-223
Olaparib + Radium-223For patients with bone metastases, it has been theorized that the combination of a PARP inhibitor and radium-223 may have synergistic mechanisms of action. PARP inhibitors have shown efficacy as radiosensitizing agents which may promote the efficacy of radium-223, an α-emitting radioisotope that induces DNA double-strand breaks leading to cell death. This formed the foundation for the COMRADE trial, an open-label, multi-center, phase 1/2 study trial to test the safety and efficacy of radium-223 and olaparib. This trial included men with mCRPC who had ≥2 bone metastases without evidence of concurrent visceral metastases or lymphadenopathy > 4 cm.
The phase 1 portion of the study employed a 3+3 dose escalation design with fixed-dose radium-223 (55 kBq/kg IV every 4 weeks x 6) and escalating doses of olaparib. The dose level 1 (DL1) for was olaparib 200 mg PO BID while DL2 was 300 mg PO BID. In phase 1, the primary objective was to determine the recommended phase 2 dose (RP2D) for the randomized portion of the study, which was found to be 200 mg BID for olaparib. No dose limiting toxicities were observed at either DL1 or DL2. However, 5 of 6 patients enrolled at DL2 required dose reduction. Assessing secondary objectives, the authors found that the PSA response and alkaline phosphatase response rates were 16.7% (n=2) and 67% (n=8), respectively. At a median follow-up of 6.5 months, the 6 months rPFS was 58%, and the 12 months OS was 56%. Based on these results, the investigators concluded that olaparib can be safely combined with radium-223 at the RP2D of 200 mg orally twice daily with fixed dose radium-223.8
Niraparib + Radium-223
Utilizing a similar treatment strategy to that seen in the COMRADE trial, the combination of niraparib and radium-223 was evaluated in the phase 1b trial, NiraRad. This trial included 30 men with progressive mCRPC following ≥1 line of an ARPI and had evidence of bone metastases without bulky visceral disease and no documented BRCA1/2 alterations. The niraparib dose was escalated in combination with standard dosing of Radium-223 using a time-to-event continual reassessment method. The investigators determined that for patients with prior chemotherapy exposure, the maximum tolerated dose (MTD) for niraparib was 100 mg, whereas the MTD for chemotherapy-naïve patients was 200 mg. The median rPFS for all patients included in analysis was 7.1 months with an estimated 6-month rPFS of 51%.9
PARP Inhibitors + 177Lu-PSMA-617
177Lu-PSMA-617 delivers significant beta radiation to PSMA-expressing tumors causing single strand DNA breaks, which are typically repaired by PARP-dependent pathways. Blocking the PARP enzyme could have a synergistic mechanism of action by converting DNA single strand breaks to lethal double strand breaks via replication fork collapse. In the LuPARP trial presented at ASCO 2023, the investigators hypothesized that olaparib would promote the radiosensitization of 177Lu-PSMA-617, resulting in intensification of DNA damage and, thus, improved efficacy.
The LuPARP phase 1 trial schema was as follows:
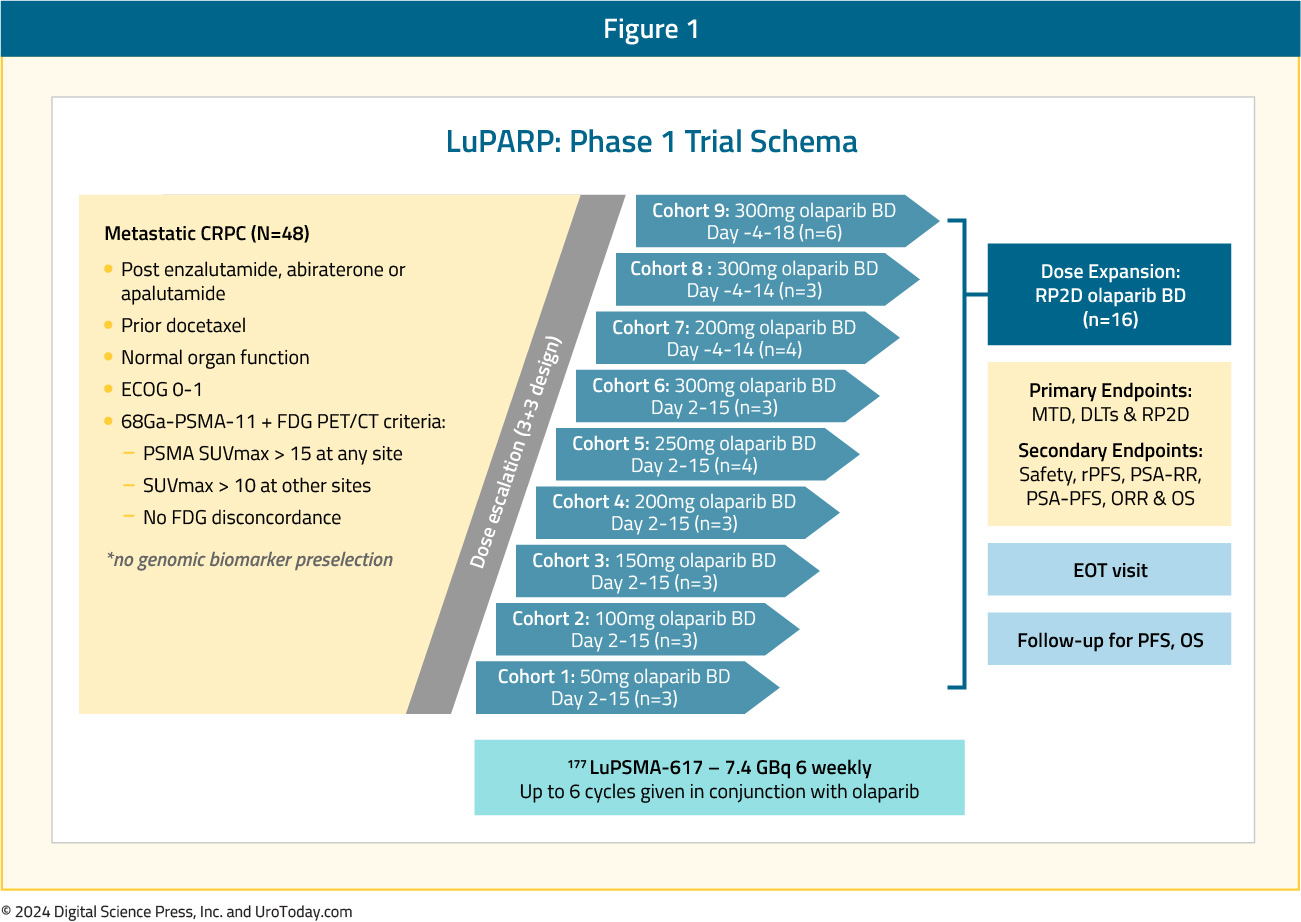
This trial included 48 patients with mCRPC, and all eligible patients had received a prior ARPI and docetaxel. All patients underwent a 68Ga-PSMA-11 plus an FDG-PET/CT with the following inclusion criteria:
- PSMA SUVmax >15 at any site
- SUVmax >10 at other sites
- No FDG discordance
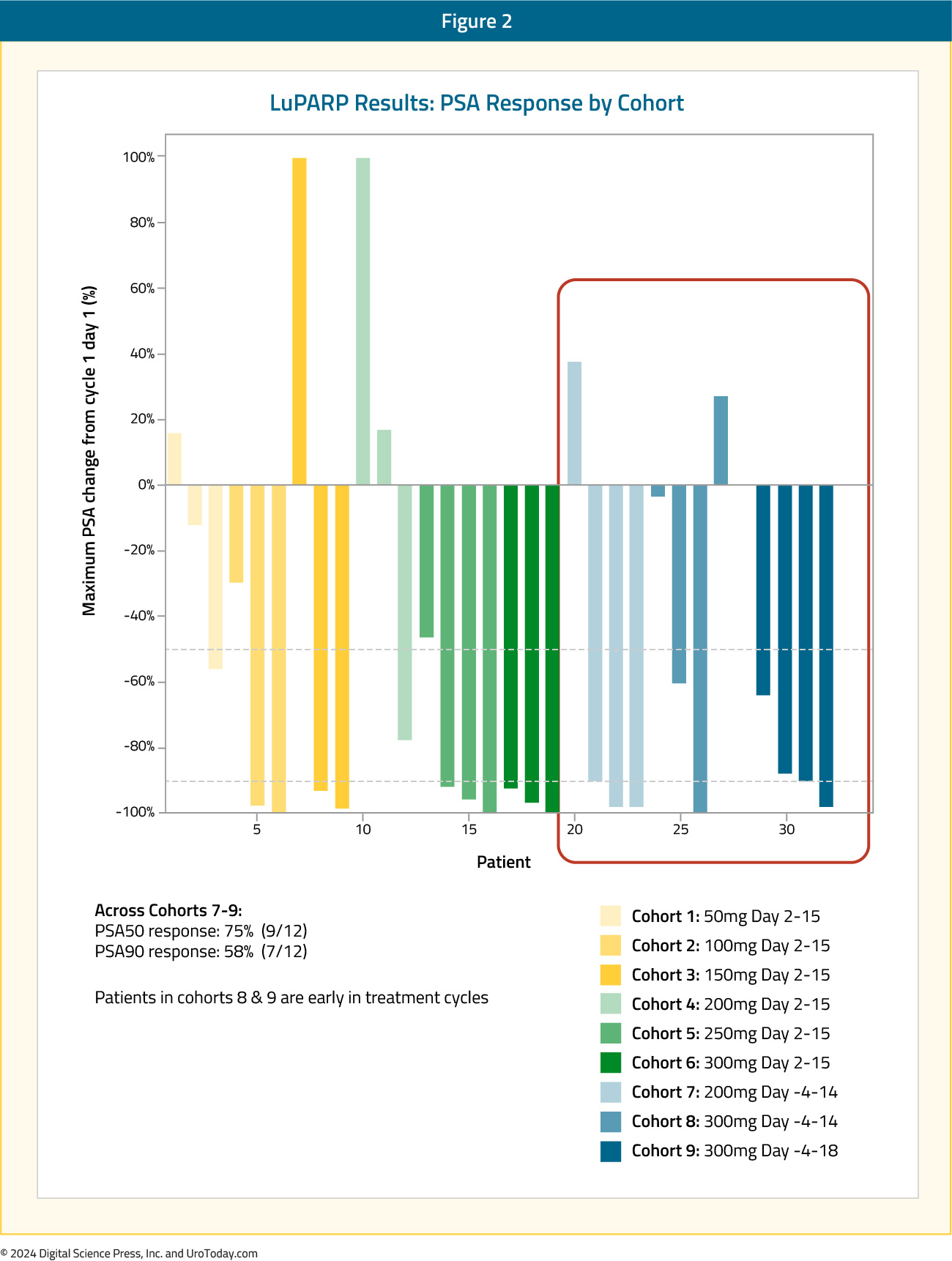
From an efficacy standpoint, 177Lu-PSMA-617 in combination with olaparib demonstrated promising activity: in the overall cohort (i.e., Cohorts 1 to 9), the PSA50 and PSA90 response rates were 66% and 44%, respectively. The objective response rate (ORR) by RECIST v1.1 criteria was 78%.10 Compared to the results of the TheraP and VISION trials, the PSA50 responses were identical to those from TheraP (66%) and higher than those in VISION (46%).11,12 The PSA90 response of 44% in LuPARP was slightly higher than that in TheraP (38%).
Moreover, early results from Cohorts 7-9 were promising with PSA50 and PSA90 responses of 75% and 58%, respectively. However, results from this Phase 1 trial are not designed, nor powered, to assess efficacy outcomes.
PARP Inhibitors + Immune Checkpoint Inhibitors
While immunotherapy has shown limited success in the mCRPC disease space, it is hypothesized that the increased cellular DNA damage induced by PARP inhibitors may lead to increased immune priming and subsequently promote immune cell infiltration. This has served as the rationale for emerging trials of combination PARP inhibitors and immune checkpoint inhibitors.Rucaparib + Nivolumab
The CheckMate 9KD trial has evaluated the combination of rucaparib and nivolumab in two cohorts:
- Cohort A1: Post-chemotherapy mCRPC (1–2 taxanes and ≤2 ARPIs)
- Cohort A2: Chemotherapy-naïve mCRPC (Received prior ARPI)
Among patients in Cohort A1 (n=58), the ORR was 10.3% in the overall cohort. Superior ORRs were observed in the HRD-positive (17.2%) and BRCA1/2-positive tumors (33.3%). PSA50 responses were observed in 12% of patients in the overall cohort, compared to 18% and 42% of HRD-positive and BRCA1/2-positive tumors, respectively. Median rPFS ranged between 4.9 and 5.8 months, whereas OS ranged between 13.9 and 15.4 months.

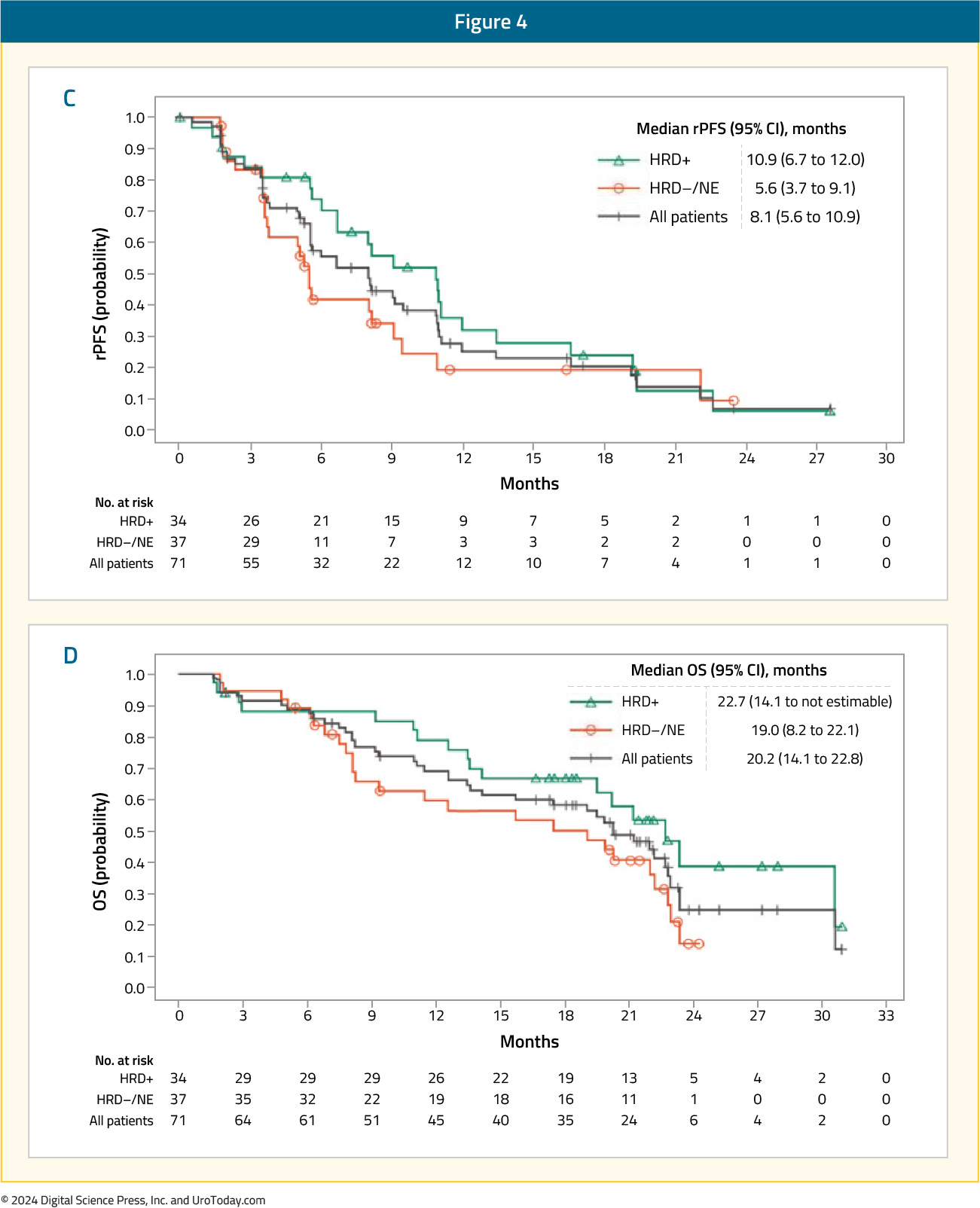
As expected, response rates and survival outcomes were superior in the less heavily pre-treated Cohort A2 (n=39). The ORR was 15.4% in the overall cohort, with ORRs of 25% and 33.3% in the HRD-positive and BRCA1/2-positive tumors, respectively. PSA50 responses were observed in 27.3% of patients in the overall cohort, compared to 42% and 85% of HRD-positive and BRCA1/2-positive tumors, respectively. Median rPFS ranged between 8.1 and 10.9 months, whereas OS ranged between 20.2 and 22.7 months.
In cohorts A1 and A2, respectively, the most common any-grade and grade 3–4 treatment-related adverse events were nausea (41%) and anemia (14–21%). Approximately 25% of patients discontinued treatment secondary to adverse events.13
Olaparib + Pembrolizumab
Cohort A of the phase 1b/2 KEYNOTE-365 study enrolled patients with molecularly unselected, docetaxel-pretreated mCRPC whose disease progressed within 6 months of screening. In this trial, 102 patients received pembrolizumab 200 mg IV every 3 weeks + olaparib 400 mg capsule or 300 mg tablet orally twice daily. Patients could have received one chemotherapy agent other than docetaxel for mCRPC and ≤2 ARPIs. The primary endpoints were PSA50 response rates, ORR, and safety.
A PSA50 response was observed in 15% of patients. The confirmed ORR was 8.5% (5 partial responses) among patients with measurable disease.

The median rPFS was 4.5 months, and the median OS was 14 months. Treatment-related adverse events were observed in 91% of patients. Grade 3–5 events occurred in 48% of patients (6% deaths), most commonly anemia (27%), fatigue (6%), and neutropenia (5%).14
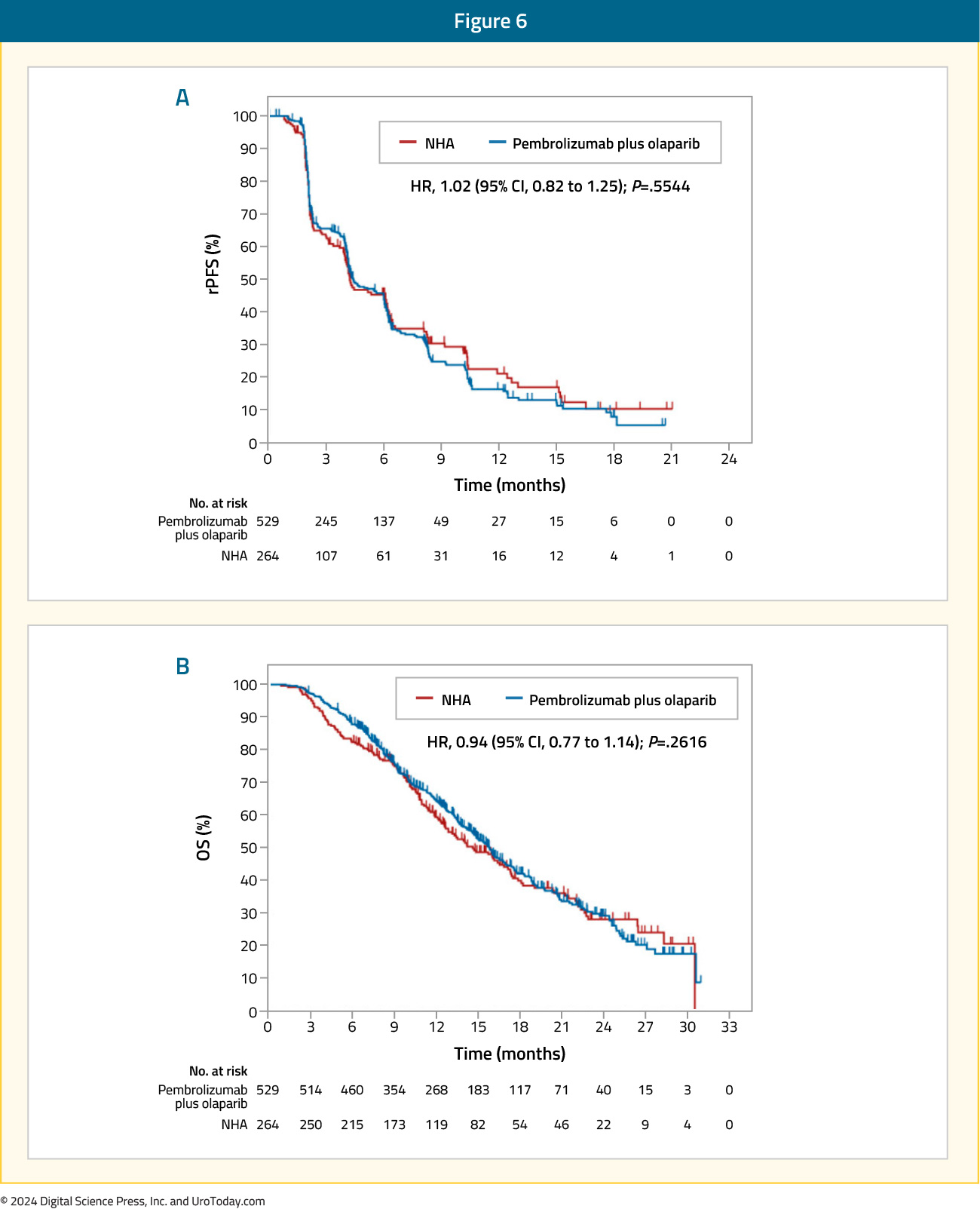
This combination of olaparib + pembrolizumab was next assessed in the open-label, phase III KEYLYNK-010 trial that randomized mCRPC patients that had progressed on one prior ARPI and docetaxel in a 2:1 fashion to olaparib + pembrolizumab versus the alternate ARPI (i.e., if had received abiraterone, given enzalutamide and vice versa). The dual primary endpoints were rPFS and OS. This trial included 793 patients of whom 529 and 264 were randomized to olaparib + pembrolizumab and an alternate ARPI, respectively. There was no significant difference in rPFS (median: 4.4 versus 4.2 months; HR: 1.02, 95% CI: 0.82 – 1.25, p=0.55) or OS between the two treatment arms (median 15.8 versus 14.6 months; HR: 0.94, 95% CI: 0.77 – 1.14, p=0.26).
Grade 3 treatment-related adverse events were more common with olaparib + pembrolizumab (35% versus 9%), with events leading to treatment discontinuation occurring in 11% and 1.6% of patients in the intervention and control arms, respectively. The most common grade ≥3 adverse events with olaparib + pembrolizumab were anemia (20%), fatigue (3%), and asthenia (2.3%).15
Olaparib + Durvalumab
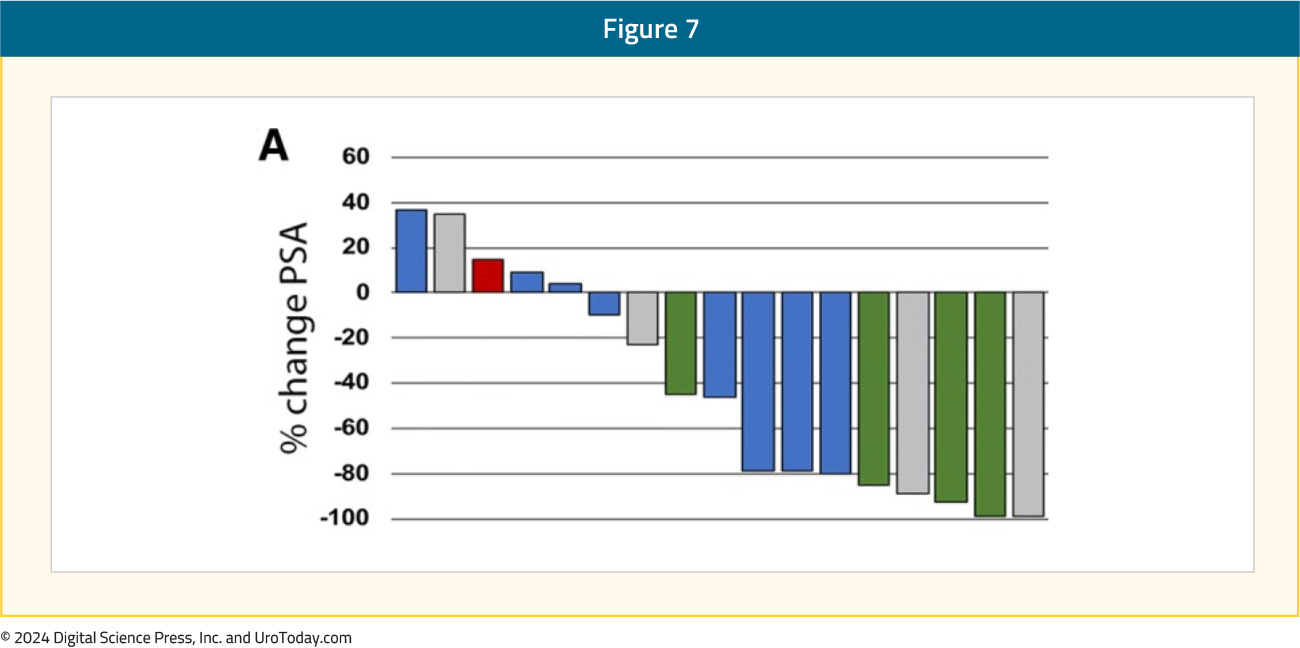
In a single arm phase II trial, the combination of durvalumab 1,500 mg IV every 4 weeks and olaparib 300 mg twice daily was evaluated in 17 mCRPC patients with disease progression following prior ARPI. Overall, 9/17 (53%) patients had a PSA50 response, with 4 of these 9 patients having a radiographic response. The median rPFS of patients with DDR gene alteration was 16.1 months, with a 12-months PFS probability of 83.3%, compared to 36.4% in those without mutations (p=0.031). The most common treatment-related grade 3 or 4 adverse events were anemia (24%), lymphopenia (12%), infection (12%), and nausea (12%).16
Talazoparib + Avelumab
The JAVELIN PARP Medley trial is a phase 1b/2 basket trial evaluating the combination of talazoparib and avelumab in patients with advanced solid tumors, including mCRPC patients with and without HHR alterations (n=21). Patients received avelumab 800 mg every 2 weeks plus talazoparib 1mg once daily. In the overall cohort, PSA responses were observed in 2/21 patients, and in the HRR positive mCRPC cohort, the ORR was 11.1%.17
PARP Inhibitors + Bipolar Androgen Therapy
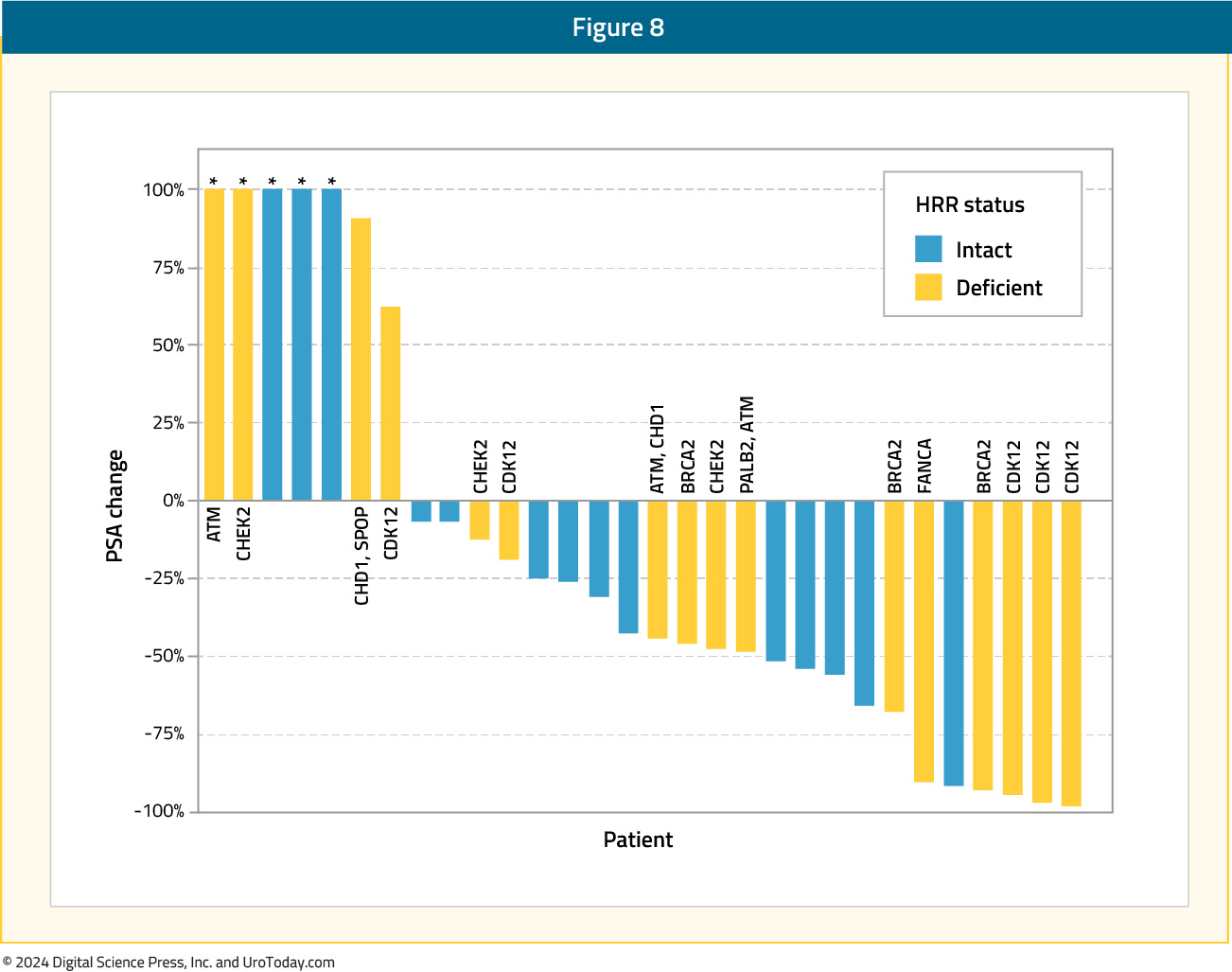
Prostate cancer cells can develop resistance to androgen ablation through an adaptive marked upregulation of androgen receptors over time in response to a low-androgen milieu. This upregulation can make these cells vulnerable to supraphysiologic testosterone exposure. Bipolar Androgen Therapy (BAT) has been proposed as a technique to overcome AR therapeutic resistance. Rapid cycling between polar extremes of supraphysiologic and near-castrate serum testosterone in asymptomatic men with mCRPC has proven to be safe and effective.18Supraphysiologic androgen levels have been shown to induce double-strand DNA breaks and suppress the expression of genes involved in the DNA repair process.19,20 This has served as the rationale for evaluating the combination of olaparib and BAT in a single arm phase II trial. Thirty-six patients with mCRPC and disease progression following abiraterone and/or enzalutamide received olaparib 300 mg twice daily plus BAT (testosterone cypionate/enanthate 400 mg every 28 days with ongoing androgen deprivation). A PSA50 response was observed in 11/36 patients (31%) at 12 weeks, and the median rPFS in the intent-to-treat cohort was 13 months. The most frequently observed treatment-related adverse events were gastrointestinal related and fatigue. Five patients had grade ≥3 treatment-related adverse events, including one stroke (Grade 4) and one myocardial infarction (Grade 5).21
PARP Inhibitors + Chemotherapy
The combination of the low dose oral PARP inhibitor veliparib (ABT-888) and temozolomide for docetaxel pre-treated mCRPC patients was evaluated in a single-arm, open-label, pilot study published by Hussain et al. in 2014. This trial included 26 patients with a median baseline PSA of 170 ng/ml. A PSA response was observed in 2 patients (8%), with a further 13 having stable PSA levels. The median PFS was 9 weeks, and the median OS was 40 weeks. Grade 3/4 adverse events occurred in >10 % of patients include thrombocytopenia (23 %) and anemia (15 %).22PARP Inhibitors + Targeted Therapies
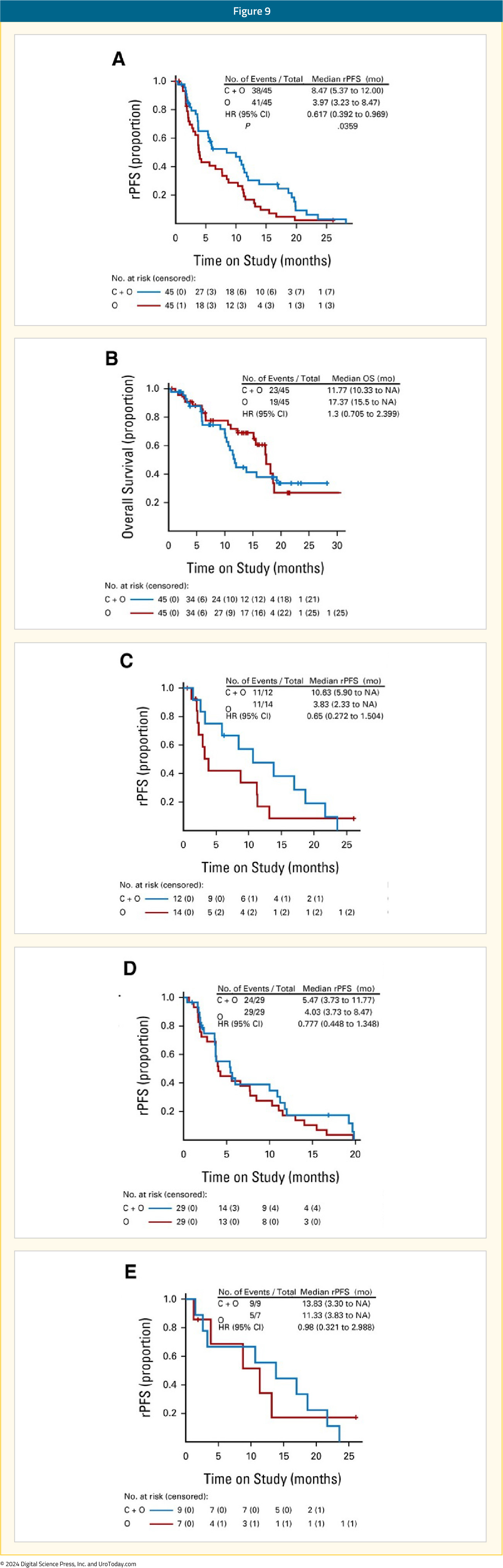
Olaparib + CediranibCediranib is a pan-vascular endothelial growth factor receptor inhibitor that suppresses the expression of HRR genes and increases sensitivity to PARP inhibition in preclinical models.23 In an open-label phase II trial, patients with progressive mCRPC were randomly assigned to receive either cediranib 30 mg once daily plus olaparib 200 mg twice daily versus olaparib 300 mg twice daily alone. In the intention-to-treat cohort of 90 patients, the median rPFS was 8.5 months in the combination arm versus 4 months in the PARP inhibitor monotherapy arm (HR 0.62; 95% CI: 0.39–0.97, p=0.036). Among patients with HRR-deficient mCRPC, the median rPFS was 10.6 months with combination treatment versus 3.8 months with olaparib monotherapy. In the subset of patients with BRCA2-mutated mCRPC, median rPFS was 13.8 months in the combination arm versus 11.3 months in the olaparib only arm. Grade 3–4 adverse events occurred in 61% of patients in the combination arm, compared to 18% of patients in the monotherapy arm.24
Olaparib + Ceralasterib
In an in vitro study, the combination of olaparib and the ataxia telangiectasia and Rad3-related protein (ATR) inhibitor, ceralasterib, was shown to selectively cause cell death in ATM-deficient cells.25 This served as the basis for the TRAP trial, a two-cohort study of mCRPC patients with HRR mutations (BRCA1/2 or ATM; n=35) and another without HRR mutations (n=12). All patients had progressed on ≥1 prior mCRPC therapy with no prior PARP inhibitors or platinum chemotherapy. In this study, olaparib was administered twice daily at a standard dose, and ceralasterib was administered daily on days 1¬–7 of a 28-day cycle. The primary endpoint was disease response (confirmed PSA50 or RECIST response). The response rate in the HRR cohort was 33%, compared to 11% in the HRR negative cohort, including 21% of patients experiencing a grade 3 treatment-related adverse event (no grade 4–5 events).26

Conclusions and Future Trials
PARP inhibitors are an exciting class of drugs with a unique mechanism of action that lends itself to potential synergistic combinations with other classes of drugs. To date, the only combination to receive regulatory approval is that of PARP inhibitors + ARPIs; however, numerous exciting combinations continue to emerge. Additionally, given their success in the mCRPC disease space, there is increased interest in evaluating such combinations in earlier disease stages, including the high-risk localized and the metastatic hormone-sensitive settings. Summarized in the table below are select trials of PARP inhibitor combination therapy across the prostate cancer spectrum.

Published March 2024


