Imaging plays a significant role in the diagnosis and management of prostate cancer. While transrectal ultrasound and, subsequently, multiparametric magnetic resonance imaging (mpMRI) have become well-established modalities in the initial diagnosis of prostate cancer, numerous techniques for the distant staging of prostate cancer have all suffered from significant limitations.
Evolution of Next-Generation Imaging
Plain films have limited sensitivity for bone metastases (<50%)1 and require extensive bone mineral loss (exceeding 30-50%) before such changes are radiographically apparent.2 99mTc-methylene diphosphonate radionuclide bone scintigraphy (i.e. bone scan) offered improved sensitivity (86%) for detection of bone metastases compared to plain films,1 however its specificity remained low with false positives secondary to benign etiologies such as previous fractures proving problematic. Cross sectional modalities in the form of computed tomography (CT) and MRI offer the additional ability of detecting nodal and visceral metastases. However, such modalities rely on size and anatomic abnormalities for detection of metastases, which limits their respective sensitivities in low volume settings.1
In more recent years, combined morphologic and molecular imaging modalities have emerged as novel alternatives for the detection and monitoring of prostate cancer. Traditional positron emission tomography (PET) imaging using fluorodeoxyglucose (FDG) has proven ineffective in prostate cancer staging secondary to the relatively low metabolic activity (i.e., glycolysis) associated with prostate cancer.3 The next tracers to be evaluated in this setting were 11C and 18F-radiolabelled choline, a phospholipid precursor selectively taken up by rapidly dividing cancer cells. 11C-choline PET/CT was shown to have superior sensitivity to conventional imaging for the detection of pelvic nodal recurrence in patients with biochemical recurrence,4 and was subsequently approved by the U.S. Food and Drug Administration (FDA) for this indication in 2012. However, this technique was still limited by poor performance at low prostate-specific antigen (PSA) levels and the 11C-choline tracer a short half-life of 20 minutes that necessitated on-site production by a cyclotron, limiting potential wide-scale distribution.5 Use of 18F-labelled choline prolonged the half-life to 110 minutes, however the performance limitations of this imaging modality remained significant.
Fluciclovine PET/CT (Axumin® PET/CT) was the next imaging modality to gain FDA approval in this disease space in 2016. Fluciclovine, an 18F-labelled analog of L-leucine that is selectively taken up by amino acid transporters upregulated on the surface of prostate cancer cells, has the advantage of limited renal uptake and no activity in the urinary tract.6 Fluciclovine PET/CT showed improved performance in the detection of recurrence prostate cancer in patients with biochemical recurrence following local therapy, with a sensitivity of 90% and specificity of 40%.7 Importantly, when compared with choline PET/CT, this modality demonstrated lower false-negative and false-positive rates in patients with biochemical recurrence.8,9 However, this approach was still limited by low detection rates among patients with low PSAs (less than 1-2 ng/mL).
Since the initial reports of prostate-specific membrane antigen (PSMA)-based PET/CT in 2013,10 the field of PSMA-based imaging has seen significant growth. PSMA is a transmembrane protein expressed by the epithelial cells lining the proximal renal tubules, salivary glands, small bowel, as well as the prostate. Expression of this transmembrane protein is upregulated in prostate cancer cells by up to a 1000-fold. The PSMA gene is located on the short arm of chromosome 11 in a region that is not commonly deleted in prostate cancer, thus making it highly prevalent in all forms of prostate cancer, including some castrate resistant forms. Importantly, the relatively poor expression of PSMA in other organs allows for enhanced targeted imaging in prostate cancer patients.11 Previous PSMA-targeted imaging modalities such as ProstaScint had limited clinical utility secondary to limitations regarding the associated targeting of the intracellular epitope of PSMA (current PSMA-PET/CT radiotracers target the extracellular component), poor imaging characteristics of the 111In radionuclide labeling, and longer blood pool biodistribution of the antibody imaging agent.10
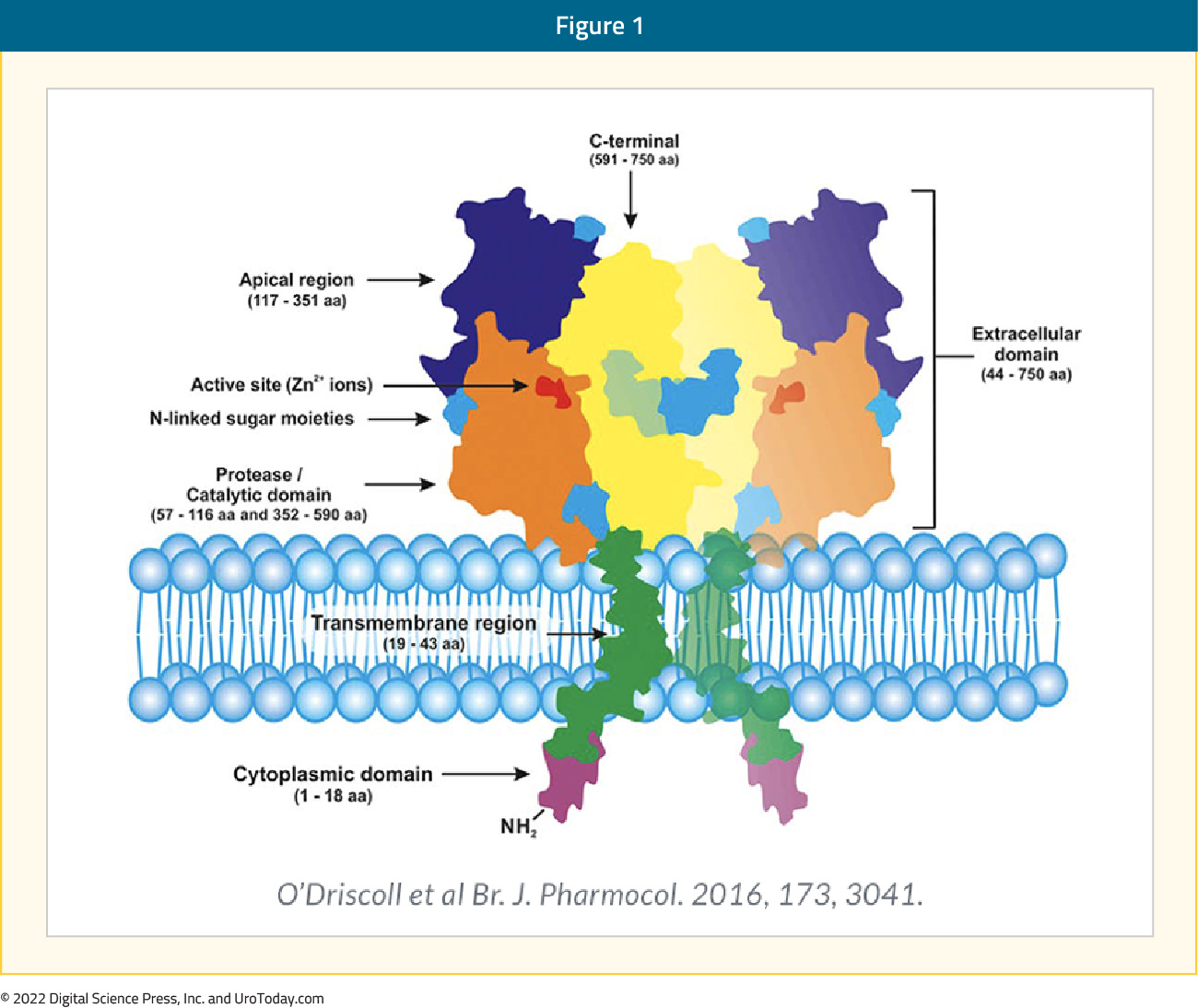
The radiotracers most commonly evaluated in the PSMA PET/CT setting are 68Ga-PSMA-11 (t½: 68 minutes) and 18F-DCFPyL (t½: 110 minutes), with additional radiotracers including PSMA-617, PSMA-1&T, PSMA-1007, and rhPSMA-7.12 68Ga PSMA-11 was FDA approved in December 2020 (with limited availability in the United States) for the initial staging of prostate cancer patients at high risk of metastases and in those with suspected recurrence based on elevated serum PSA levels. This was followed by the FDA approval of 18F-DCFPyL (Pylarify®) in May 2021 (with wide availability secondary to a longer t1/2 facilitating wider distribution).
PSMA PET/CT imaging has gained prominence across the prostate cancer diagnostic spectrum. In particular, PSMA imaging has proven utility in the assessment of the primary tumor, in staging of patients with high-risk disease, in the evaluation of patients with biochemical recurrence following local therapy, and in the assessment of patients with metastatic castration resistant disease for suitability for theranostic treatment. In this center of excellence article, we discuss the current evidence for PSMA PET-based imaging in the initial diagnosis of prostate cancer, including in the setting of patients with a known history of prostate cancer, patients that are biopsy naïve, and among men with a prior negative prostate biopsy. Other indications will be discussed in subsequent articles.
Evaluation and Diagnosis of the Primary Tumor
Although mpMRI remains a critical component of the diagnostic pathway for prostate cancer and is currently recommended prior to prostate biopsy by numerous guidelines,13 up to 25% of clinically significant prostate cancer lesions remain “MRI invisible”.14 Accordingly, PSMA PET/CT has been evaluated in this setting, both alone and in combination with mpMRI, to aid in the detection of such lesions.
Known Prostate Cancer Patients
Initial studies verified the utility of PSMA PET/CT for the detection of intra-prostatic lesions in patients with known prostate cancer, with subsequent studies evaluating its potential role in detecting intra-prostatic lesions during the work up of at-risk patients. The DeTeCT trial prospectively included 30 patients with intermediate/high risk prostate cancer who underwent 18F-DCFPyL PET/CT prior to planned radical prostatectomy. Blinded nuclear medicine physicians assessed tumor localization on PET/CT and made biopsy recommendations based on the size and PET-intensity of observed lesions. The biopsy recommendation was subsequently correlated with final histopathology in the prostatectomy specimen. Clinically significant prostate cancer was present in 28/30 (93%) of the recommended biopsy segments and the highest Gleason Score disease was present in 26/30 segments (87%). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for clinically significant prostate cancer per segment using 18F-DCFPyL-PET/CT were 61.4%, 88.3%, 68.1% and 84.8%, respectively.15
Eiber et al. compared the diagnostic performance of 68Ga-PSMA-11 PET/CT, mpMRI, and combined PSMA PET-CT/mpMRI in 53 patients with known prostate cancer. Simultaneous PET/MRI statistically outperformed mpMRI alone (area under the curve [AUC]: 0.88 vs 0.73; p < 0.001) and PET imaging alone (AUC: 0.88 vs 0.83; p = 0.002) for localization of prostate cancer. PET imaging alone was more accurate compared to mpMRI alone (AUC: 0.83 vs 0.73; p = 0.003). PET provided a high uptake ratio between malignant versus nonmalignant tissue (5.02 [range: 0.89–29.8]), but no significant correlation was seen between quantitative PET parameters and Gleason Score or PSA values.16
Biopsy Naïve Patients
PEDAL is a phase III trial comparing the diagnostic accuracy of mpMRI to 18F-DCFPyL-PET/CT among patients without a history of prostate cancer. This multicenter trial includes 235 patients from Australia and New Zealand with an indication for a prostate biopsy (elevated PSA and/or suspicious rectal exam) who had no known diagnosis of prostate cancer and no prostate biopsy or prior mpMRI within 3 years of trial inclusion. All patients underwent both imaging modalities, and ultimately 180 (76.6%) underwent a prostate biopsy (targeted + systematic). A positive MRI was defined as a PIRADS 3 or worse lesion, while the PET/CT was positive if the standardized uptake value (SUV) max was ≥7 (SUV Max 4-7: equivocal)
With regards to the primary outcome of diagnostic accuracy in detecting the presence of any prostate cancer, mpMRI demonstrated superior performance: receive operating characteristic (ROC) AUC 0.77 for MRI (95% CI: 0.70 – 0.84) versus 0.52 for 118F-DCFPyL-PET/CT (95% CI: 0.54 – 0.70). mpMRI significantly outperformed 18F-DCFPyL-PET/CT with regards to specificity (75.9% versus 55.6%) and NPV (61.2% versus 45.5%).
Could these two imaging modalities be used in conjunction to maximize the combined NPV and potentially forgo a prostate biopsy if both are negative? Of 94 patients with a negative MRI (PIRADS 0-2), 30 (31.9%) had a positive PET scan, and among these 30 patients, 24 underwent a prostate biopsy with prostate cancer diagnosed in 9 (37.5%) of 24 patients. Conversely, of the 50 patients with negative PET scan findings, 8 (16%) had a PIRADS 4-5 lesion. Of these 8 patients, 5 (62.5%) were found to have prostate cancer on a subsequent biopsy:

Thus, clearly there is a subset of patients with “MRI invisible” prostate cancer that could be detected using 18F-DCFPyL-PET/CT, and vice versa. Using both modalities, either sequentially or in parallel, comes at a high financial cost and thus remains impractical in most, if not all, health care settings. As reporting standards of PSMA PET scan findings continue to improve and we identify a subcohort of patients who may benefit from combined imaging in the future, such financial concerns may be partially alleviated by this more selective utilization.
In a similar setting to the PEDAL trial, a prospective study comparing the performance of [F-18] FlorastaminR PSMA PET/CT with that of mpMRI for the initial diagnosis of prostate cancer was recently presented at AUA 2022. Eighty-two patients with suspicion of prostate cancer (abnormal rectal exam or PSA >3.0 ng/ml) were recruited. After [F-18] FlorastaminR PET/CT and mpMRI were both performed, a saturation (20 core) transperineal biopsy was performed. An MRI was considered positive if a PIRADS 3 lesion or worse was present. PSMA PET/CT was considered positive if the urologist who performed the prostate biopsy noted a lesion with higher uptake compared to the background level and was unrelated to physiological uptake or known pitfall. Of the 82 patients, 27 (32.9%) were diagnosed with clinically significant prostate cancer. mpMRI demonstrated an accuracy of 69.5% for clinically significant prostate cancer, which was inferior to that seen with [F-18] FlorastaminR PET/CT (70.7% - 78.0%). In addition to superior accuracy for detection of clinically significant prostate cancer, [F-18] FlorastaminR PET/CT was shown to be superior at correct localization of clinically significant disease (70.7% - 78.0% versus 68.3%).
The PRIMARY trial (ANZCTRN12618001640291) is a multicenter, phase II imaging trial that evaluated if a limited (i.e. pelvic-only) 68Ga-PSMA-11 PET/CT in combination with mpMRI can reliably discriminate men with clinically significant prostate cancer (i.e. ≥grade group 2) from those without clinically significant disease. This trial included 291 men with suspected prostate cancer (PSA<20), no previous biopsy, recent MRI within 6 months, and planned transperineal biopsy based on clinical risk and MRI. For analysis, the combination of PSMA and MRI was considered negative for PSMA negative PI-RADS 2/3 or positive for either PSMA positive PI-RADS 2/3 or PI-RADS 4/5. Of these 291 men, 162 (56%) had clinically significant prostate cancer. MRI, PSMA, and PSMA+MRI was positive in 67%, 73%, and 81% of patients, respectively. Combination PSMA and MRI, compared to MRI alone, improved the sensitivity (97% versus 83%, p<0.001) and NPV (91% versus 72%, p<0.001). However, this was at the cost of reduced specificity (40% versus 53%, p=0.001). Among all 291 patients, 56 (19%) were both PSMA and MRI negative and could have potentially avoided a biopsy at a risk of delayed clinically significant prostate cancer detection in 3.1% of patients.17
Prior Negative Biopsy
A prospective observational study published in 2018 evaluated 68Ga-PSMA-11 PET/CT in a cohort of patients with at least one negative prior prostate biopsy and persistently elevated PSA and/or Prostate Health Index (PHI) suspicious for prostate cancer. Included patients had a negative rectal examination and had either a negative mpMRI (PIRADS 2 or less) or an absolute or relative contraindication to mpMRI. Mean PSA and PHI scores were 10.5 ng/ml and 43.0, respectively. Of the 45 patients, 25 patients (55.5%) had a positive PET scan and underwent a fusion target biopsy. Overall, prostate cancer was present in 11 (44%) patients, with Gleason Score 7 or worse disease present in 5/11 patients. Importantly, 68Ga-PSMA-11 PET/CT radiographic parameters correlated with biopsy findings. Mean and median uptake values on 68Ga-PSMA-11 PET/CT (maximum SUV and the maximum-to-background SUV ratio) were significantly higher for Gleason Score 7 lesions compared to Gleason score 6 or benign lesions (p<0.001). On ROC analysis, a maximum SUV of 5.4 and a maximum-to-background SUV ratio of 2 discriminated clinically relevant prostate cancer with 100% overall sensitivity in each case, and 76% and 88% specificity, respectively.18
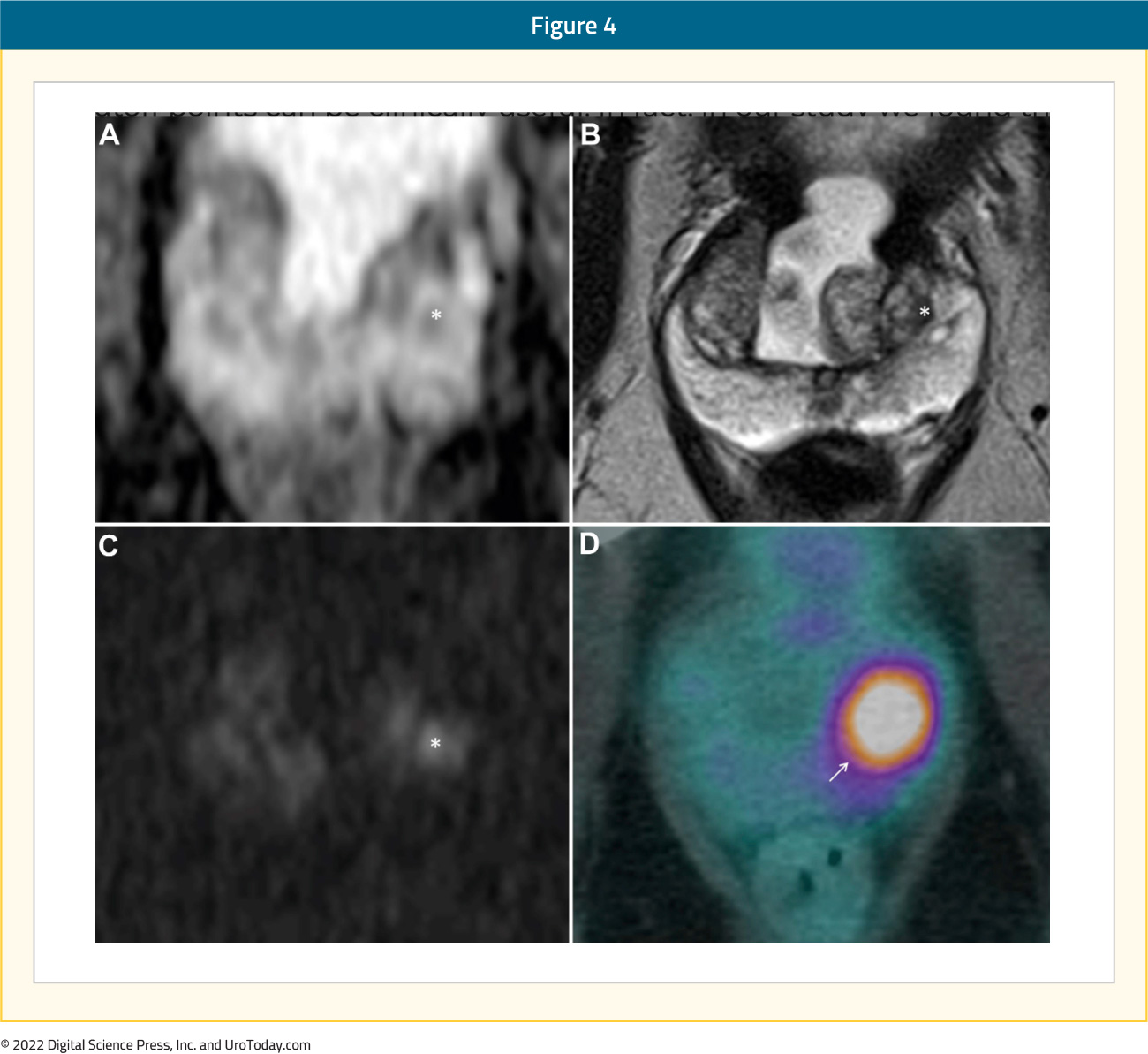
At AUA 2022, Kupperman et al. presented results of a prospective trial based out of UCLA that evaluated intra-prostatic PSMA focal uptake as a target for biopsy detection using 68Ga-PSMA PET/CT-US guided fusion. This trial included 13 patients with a prior negative biopsy (10 MRI-guided) but continued with clinical suspicion for prostate cancer and had focal uptake of PSMA in the prostate. Prostate cancer was detected in 9/13 patients (70%), with Grade Group 2, 3, and 4 diseases detected in 1, 6, and 2 patients, respectively. The median SUVmax was 6 in PSMA lesions returning <GG1 findings versus 8.2 in men with clinically significant prostate cancer. Furthermore, PSA density was higher in men with clinically significant prostate cancer (0.25 versus 0.20). The authors concluded that prostate biopsy targeting PSMA “hot spots” via PET/CT-US fusion may detect clinically significant prostate cancer missed by MRI-guided biopsy, with future larger scale studies needed to validate this.
Conclusions
PSMA PET imaging has evolved over the last decade as the next generation imaging modality of choice secondary to its improved accuracy for detecting lesions at low PSAs. Although mpMRI and transrectal ultrasound represent the mainstay for imaging for men with suspected and/or confirmed localized disease, recent trials suggest there may be utility for PSMA PET imaging in this context. However, the utility of PSMA PET imaging for evaluation of the primary tumor needs to be confirmed in larger trials, in addition to assessment with cost-effectiveness studies.
Written by:
- Rashid Sayyid, MD MSc, University of Toronto, Toronto, ON
- Zachary Klaassen, MD MSc, Medical College of Georgia, Augusta, Georgia, USA


