68Ga-based PSMA PET/CT
The growing popularity in this setting is secondary to multiple trials demonstrating its superior performance compared to conventional imaging. The seminal data supporting the role of 68Ga-PSMA imaging in for this indication, published in 2020, comes from the proPSMA study, a multi-center, two-arm randomized controlled trial of men with histologically confirmed prostate cancer who were being considered for curative intent radical prostatectomy or radiotherapy. To be eligible for inclusion, men must have had at least one high-risk factor including PSA ≥ 20 ng/mL, ISUP grade group 3-5, or clinical stage T3 or greater. Following enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging consisting of bone scan and CT or 68Ga-PSMA-11 PET/CT. In patients who had fewer than three unequivocal sites of metastasis, cross-over imaging for confirmation was performed within 14 days. Confirmatory testing following imaging was performed at the discretion of the treating physician and included biopsy confirmation.
The primary study outcome was the accuracy of first-line diagnostic imaging for identifying either pelvic nodal or distant metastatic disease. Accuracy was reported using the AUC of the ROC curve. The reference standard was a composite panel of histopathology, imaging, and biochemistry at 6-month follow-up.
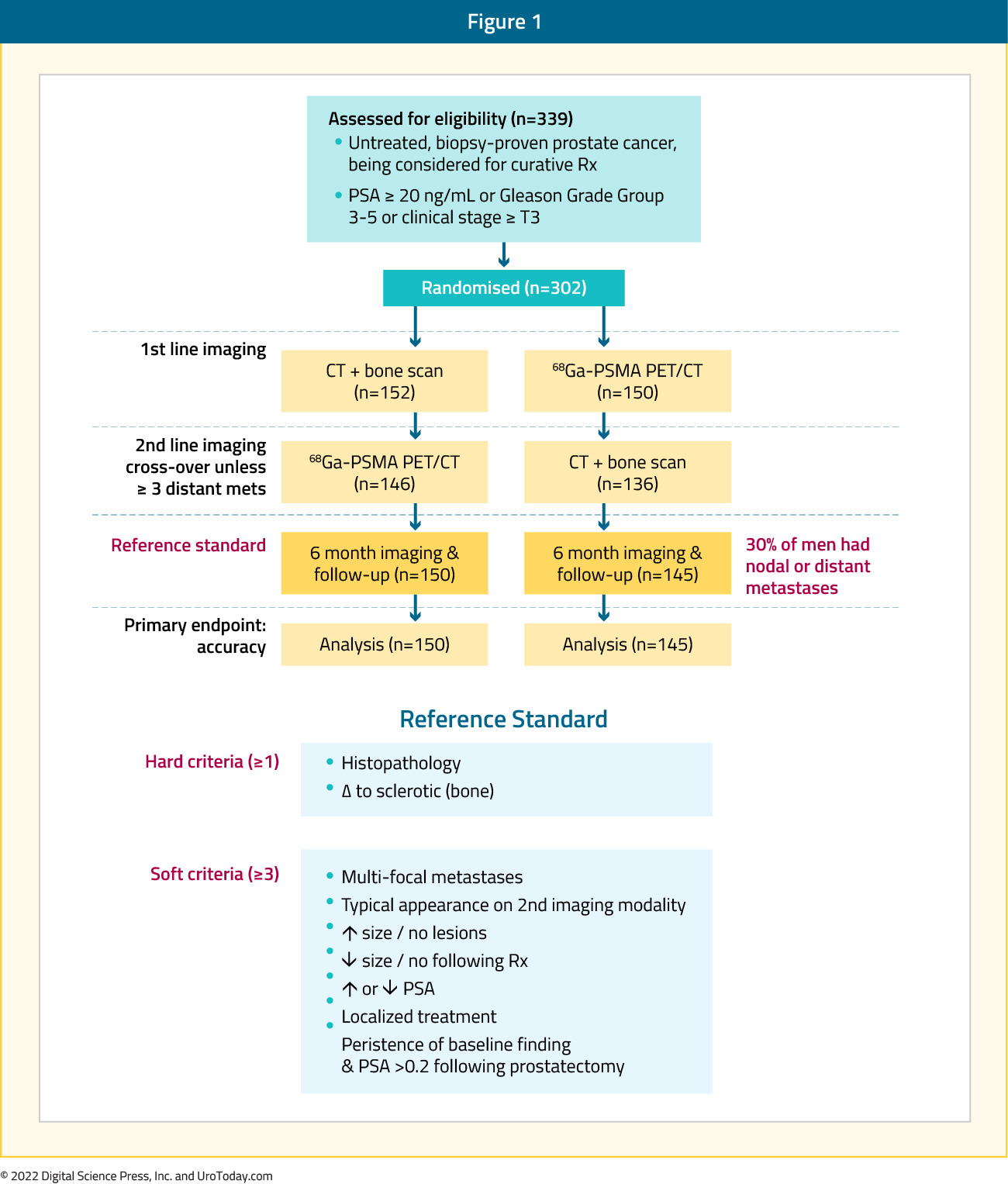
Between 2017 and 2018, 302 patients were randomized to either conventional imaging (n = 152) or 68Ga-PSMA-11 PET/CT (n = 150). As planned, the study cohort exhibited high-risk disease features: 293 (97.0%) men had ISUP grade 3 or higher, 65 (21.5%) had PSA 20 ng/mL or higher, and 82 (27.2%) had clinical stage T3 or T4 disease. Of note, 96% (n = 146) of men assigned to conventional imaging underwent subsequent second line PSMA PET-CT. Of 295 (98%) men with follow-up, 87 (30%) had pelvic nodal or distant metastatic disease. With regards to the primary outcome, PSMA PET/CT had a 27% absolute greater AUC for accuracy compared to conventional imaging (95% CI for difference: 23 – 31%): 92% (95% CI: 88 – 95%) vs. 65% (95% CI: 60 – 69%):
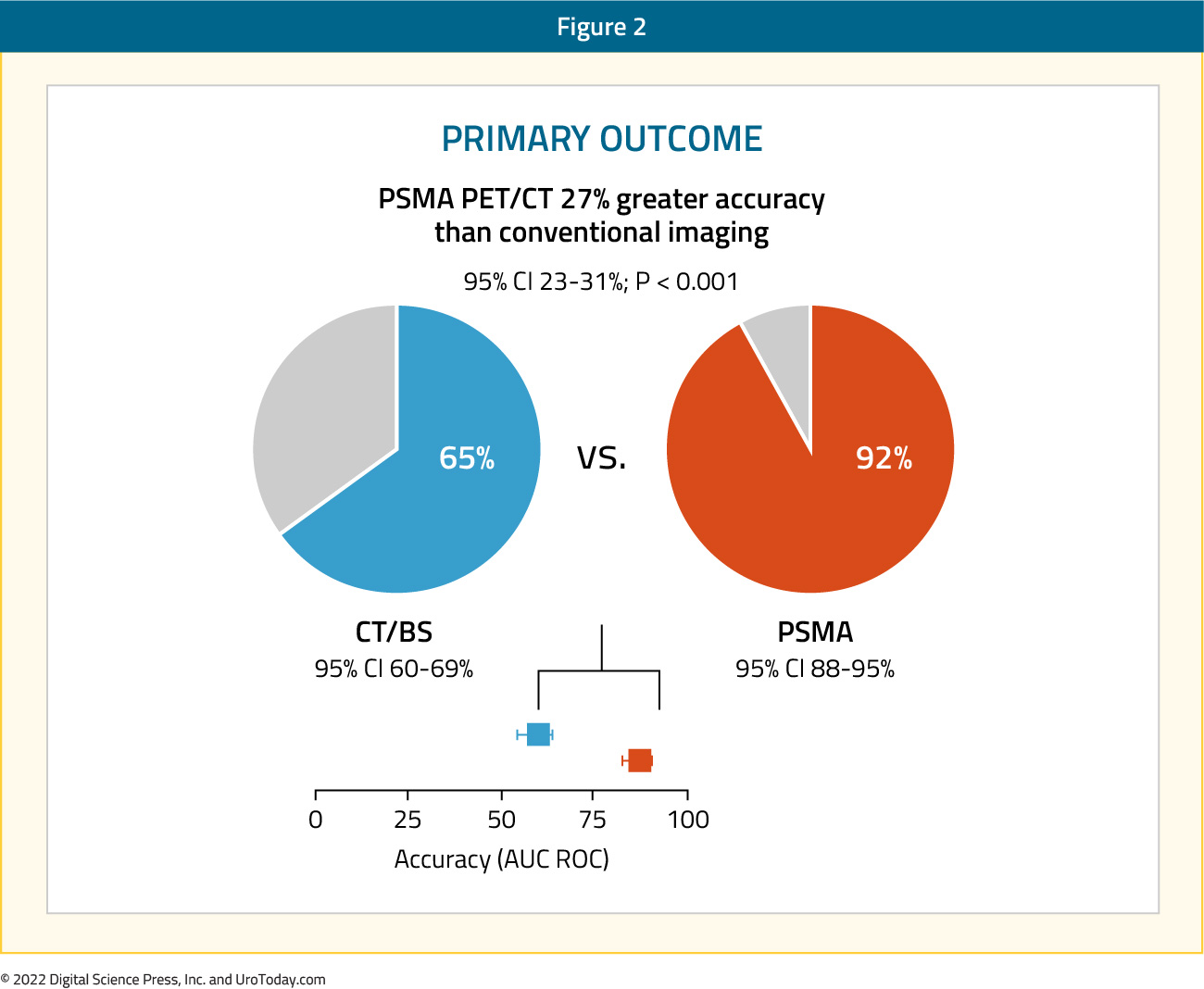
Conventional imaging had both a lower sensitivity (38% vs. 85%) and specificity (91% vs. 98%). Subgroup analyses by site of metastasis demonstrated the superiority of PSMA PET/CT for pelvic nodal (AUC: 91% versus 59%) and distant metastases (AUC: 95% versus 74%).
Imaging findings led to changes in management (treatment intent, modality, or delivery) in 28% of men in the PSMA PET/CT arm versus 15% of men in the conventional imaging arm. Following first-line PSMA PET/CT, 20 (14%) of 148 patients were directed from curative to palliative-intent treatment, 11 (7%) had a change in radiotherapy technique, and 11 (7%) in surgical technique. Notably, conventional imaging was associated with a higher radiation dose (19.2 mSv compared to 8.4 mSv; absolute difference 10.9 mSv, 95% CI for difference: 9.8 – 12.0 mSv). PSMA PET-CT was not associated with any adverse events and reporter agreement was high for both nodal (kappa=0.87, 95% CI: 0.81 – 0.94) and distant metastatic disease (kappa=0.88, 95% CI: 0.84 – 0.92).1
While the results of the proPSMA trial demonstrated potential clinical advantages, one of the immediate questions that arose what whether the use of 68Ga-PSMA-11 PET/CT was cost effective in this setting? Ad hoc cost-effectiveness analysis from the proPSMA trial demonstrated that routine use of 68Ga-PSMA-11 PET/CT in this setting saved AUD$959 per additional accurate detection of nodal disease and AUD $1,412 per additional accurate detection of distant metastases.2 However, it bears note that the cost of 68Ga-PSMA-11 PET/CT at the time of report publication was AUD $1,203 compared to AUD$ 1,412 for conventional imaging. Given that the average cost of a PET scan in the United States is USD $5,750 (range: 1,250 – 9,225), the results of this cost-effectiveness analysis may not be translatable to different healthcare systems at the current time.
In 2021, Hope et al. published results of a multicenter, single-arm, open-label phase 3 trial evaluating 68Ga-PSMA-11 PET/CT for the detection of pelvic nodal metastases in patients with intermediate and high-risk prostate cancer (at least one of the following: PSA >10 ng/mL, ≥cT2b, Gleason Score ≥7). The reference standard was histopathology obtained at time of pelvic lymph node dissection. Of 764 men initially recruited who underwent a 68Ga-11-PSMA PET/CT, 277 subsequently underwent a radical prostatectomy with pelvic lymph node dissection. Pathologic nodal involvement was present in 75/277 patients (27%). The sensitivity, specificity, positive predictive value, and negative predictive value for pelvic nodal metastases were 40.0% (95% CI: 34 – 46%), 95.9% (95% CI: 92 – 97%), 75.0% (95% CI: 70.0 – 80.0%), and 81.0% (95% CI: 76.0 – 85.0%), respectively. The limitations of PSMA PET/CT for detecting micrometastatic deposits was highlighted with true-positive lymph nodes detected having a mean size of 1.1 cm, compared to 0.6 cm for false-negative lymph nodes.3
In the same year, van Kalmthout et al. evaluated the diagnostic performance of 68Ga-PSMA-11 PET/CT in patients with newly diagnosed prostate cancer who had a negative bone scan and a greater than 10% risk for lymph node involvement (Memoria Sloan Kettering Cancer Centre nomogram) prior to planned radical prostatectomy with extended pelvic lymph node dissection. This single arm trial included 103 patients, of whom 97 underwent extended pelvic lymph node dissection (chosen reference standard). Lymph node involvement was noted in 41 patients (42.3%) of the total cohort. 68Ga-PSMA-11 PET/CT was positive in 17/41 patients for a sensitivity of 41.5% (95% CI: 26.7 – 57.8%). The specificity, positive predictive, and negative predictive values were 90.9% (95% CI: 79.3 -96.6%), 77.3% (95% CI: 54.2 – 91.3%), and 67.6% (95% CI: 55.6 – 77.7%), respectively. A PET/CT-based change of treatment was observed in 12.6% of the patients.4
Notable differences in the performance characteristics of 68Ga-PSMA-11 PET/CT in these two trials by Hope et al. and van Kalmthout et al versus those seen in proPSMA are likely secondary to differences in the reference standards used - pathologic lymph node staging in the latter two trials (Hope and van Kalmthout) versus a composite of histopathology, imaging, and biochemistry at 6-month follow-up in proPSMA. Only 66% of men in the proPSMA trial had a lymphadenectomy. As such, there was significant reliance on interval imaging findings at six months, at which time small metastatic deposits (<5 mm), if present, might have still not been visible radiographically. This phenomenon would artificially elevate the sensitivity and negative predictive value of PSMA PET/CT at time of staging.
18F-DCFPyL-based PSMA PET/CT imaging
The next tracer to be evaluated in this setting was 18F-DCFPyL, which is also FDA approved for this indication. In 2021, results of the Observe Prostate Cancer with Accuracy (OSPREY) trial were published. This was a prospective, multicenter, multi-reader, open-label phase 2/3 trial that included two distinct cohorts:
- Cohort A: Men with newly diagnosed high-risk prostate cancer (i.e., clinical stage ≥T3a or PSA >20 ng/ml or Gleason score ≥8) planned for radical prostatectomy with lymph node dissection
- Cohort B: Men with presumptive radiological evidence of recurrent or metastatic prostate cancer on conventional imaging and in whom lesions were considered amenable to biopsy
All patients underwent whole-body bone imaging and contrast-enhanced CI imaging 4-6 weeks prior to 18F-DCFPyL-PET/CT, with the tracer given at a dose of 333MBq and imaging obtained one to two hours thereafter. Importantly, images were independently evaluated by three blinded nuclear medicine physicians. For cohort A, the reference standard was histopathologic analysis of the lymph nodes obtained at time of the extended template dissection (external and internal iliac, obturator fossa). The co-primary endpoints of the study were the patient-level sensitivity and specificity for detection of pelvic lymph node metastases. Secondary endpoints in cohort A included the positive and negative predictive values, detection of extrapelvic (M1) disease, and detection of the primary tumor within the prostate.
Cohort A included 268 patients, with a median age of 65.0 years, median PSA of 9.7 ng/ml (range: 1.2 – 125.3), and Gleason Score 8 or worse disease in 216 patients (80.6%). 97% of men had no known nodal disease and 99% of men no known metastatic disease at time of study inclusion. Of the 268 patients, 252 had evaluable histopathology. Positive lymph nodes were identified in 62 patients (24.6%). The sensitivity and specificity of 18F-DCFPyL-PET/CT was 40.3% (95% CI: 28 – 53%) and 97.9% (95% CI: 95 – 99%), respectively. The positive and negative predictive values were 86.7% (95% CI: 70 – 95%) and 83.2% (95% CI: 78 – 88%), respectively. Primary tumor in the prostate was identified by the blinded readers in 95.2 – 99.3% of cases. Compared to conventional CT, 18F-DCFPyL-PET/CT demonstrated higher specificity (97.9% versus 65.1%), more than threefold higher positive predictive value (86.7% versus 28.3%), higher specificity (97.9% versus 65.1%), and similar sensitivity (40.3% versus 42.6%).
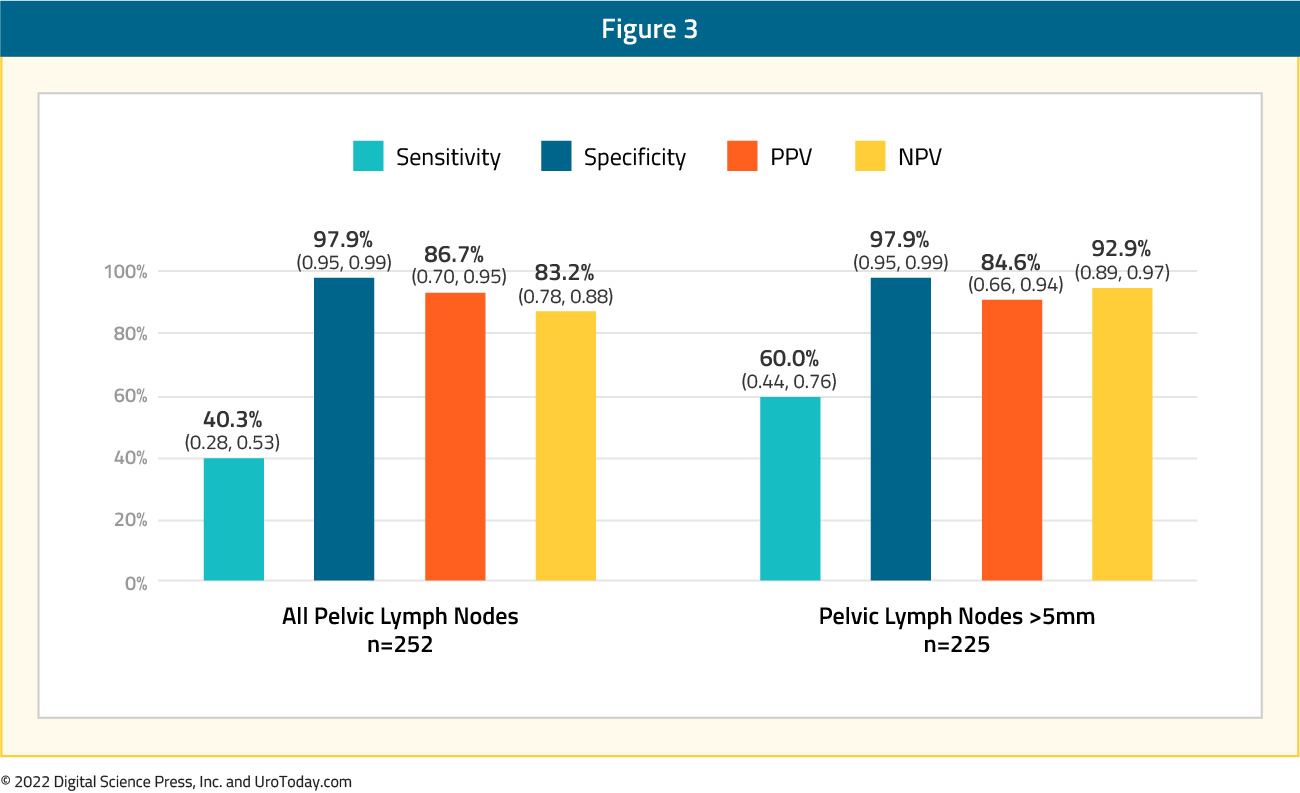
One of the main limitations of PSMA PET/CT imaging is the test’s poor ability to detect lymph node metastases <5 mm in size. Accordingly, the authors of this trial conducted a sensitivity analysis evaluating PSMA PET/CT’s performance in patients with lymph node deposits >5 mm (35/62 patients). The sensitivity and negative predictive values were significantly improved in this subcohort, with values of 60.0% (versus 40.3%) and 92.9% (versus 83.2%), respectively.5
The SALT trial, published in 2021, was a prospective multicenter trial that similarly evaluated the diagnostic performance of 18F-DCFPyL PSMA PET/CT for staging high-risk prostate cancer patients prior to treatment. This trial included 117 patients planned for robotic radical prostatectomy with extended pelvic lymph node dissection who had an ≥ 8% risk of lymph node metastases per the Memorial Sloan Kettering Cancer Centre risk nomogram. The reference standard in this trial was the available histopathologic data. Median patient was 67.0 years, and the median PSA was 10.9 ng/mL. Using EAU criteria, 43/117 (36.8%) patients had intermediate risk and 74/117 (63.3%) had high-risk prostate cancer. Median lymph node yield was 18 per patient, with 17/117 patients (14.5%) having pathological lymph node involvement. Reported performance of 18F-DCFPyL PSMA PET/CT was in keeping with the results seen in the OSPREY trial:
- Sensitivity: 41.2% (95% CI: 19.4 – 66.5%)
- Specificity: 94.0% (95% CI: 86.9 – 97.5%)
- Positive predictive value: 53.8% (95% CI: 26.1 – 79.6%)
- Negative predictive value: 90.4% (95% CI: 82.6 – 95.0%)
Again, the limitations of PSMA PET/CT in detecting LN deposits <5 mm are highlighted in this trial, whereby the median tumor size of PET-detected lymph nodes was 5.5 mm, compared to 1.5 mm for those that were not detected on imaging. Furthermore, 18F-DCFPyL PSMA PET/CT performed poorly with regards to local tumor staging (e.g. detection of ≥pT3a disease) and predicting pathologic disease grade based on peak SUV levels.6
Guideline Recommendations
Results of these trials have been acknowledged by the updated version guidelines of the European Association of Urology (EAU) and National Comprehensive Cancer Network (NCCN) Guidelines.
The updated 2022 EAU guidelines currently state:7
- “PSMA PET/CT is more accurate for staging than CT and bone scan for high-risk disease but to date no outcome data exist to inform subsequent management.” (Level of evidence: 1b).
- When using PSMA PET or whole-body MRI to increase sensitivity, be aware of the lack of outcome data of subsequent treatment changes. (Strength rating: strong)
The Prostate Cancer NCCN Guidelines Version 4.2022 include the following recommendations:8
- “Ga-68 PSMA-11 or F-18 piflufolastat PSMA PET/CT or PET/MRI can be considered for bone and soft tissue (full body) imaging.
- Because of the increased sensitivity and specificity of PSMA-PET tracers for detecting micrometastatic disease compared to conventional imaging (CT, MRI) at both initial staging and biochemical recurrence, the Panel does not feel that conventional imaging is a necessary prerequisite to PSMA-PET and that PSMA PET/CT or PSMA-PET/MRI can serve as an equally effective, if not more effective front-line imaging tool for these patients.”
- PET imaging for detection of bone metastatic disease
- Plain films, CT, MRI, PET/CT, or PET/MRI with F-18 piflufolastat PSMA, Ga-68 PSMA-11, F-18 sodium fluoride, C-11 choline, or F-18 fluciclovine can be considered for equivocal results on initial bone scan.
- Ga-68 PSMA-11 or F-18 piflufolastat PSMA PET/CT or PET/MRI (full body imaging) can be considered as an alternative to bone scan.
Moving forward, it is clear that these agents will increasingly be used for staging of patients with presumed high-risk localized disease. The data available thus far shows that this will inevitably result in changes in planned treatment for many patients. However, to date, we lack data to show how this will affect patient outcomes. Acquiring this data will be key to better defining the role of PSMA imaging.
Written by:
- Rashid Sayyid, MD MSc, University of Toronto, Toronto, ON
- Zachary Klaassen, MD MSc, Medical College of Georgia, Augusta, Georgia, USA


