Although definitive local therapy in the form of radical prostatectomy or radiation therapy with or without ADT offers excellent long-term outcomes for the majority of patients with clinically localized prostate cancer, patients with high-risk disease experience primary treatment failure rates approaching 65%.1 Disease persistence/recurrence in such patients may be restricted to the prostatic fossa, pelvic lymph nodes, non-regional lymph nodes (M1a), bones (M1b), or the viscera (M1c).
The location of disease recurrence/persistence has important clinical implications with regards to management decision making, whereby patients with local disease may be managed with curative intent, whereas those with distant disease are treated with systemic therapy. Conventional imaging modalities have failed to reliably discern disease presence/location in this setting.2-5 As such, multiple trials in this setting have evaluated the utility of PSMA PET/CT for this purpose. One of the National Comprehensive Cancer Network (NCCN) indications for using PSMA PET/CT is for staging men with biochemical recurrence after definitive primary local therapy. The following Center of Excellence article will focus on the data leading to this recommendation from the NCCN prostate cancer guidelines panel.
68Ga-based PSMA PET/CT
One of the earliest applications of PSMA PET/CT imaging was in the biochemical recurrence disease space. In the earliest published report of PSMA PET/CT in prostate cancer (2013), Afshar-Oromieh et al. evaluated 68Ga-PSMA-11 PET/CT in 37 patients with prostate cancer and evidence of progressive disease following prior treatment (33/37 radical prostatectomy, 4/37 radiation and androgen deprivation therapy (ADT)). The median patient age was 70.0 years and median PSA was 3.3 ng/ml (range: 0.01 – 148 ng/ml). Of the 37 patients, 31 (83.8 %) showed at least one lesion suspicious for cancer at a detection rate of 60 % at PSA <2.2 ng/ml and 100 % at PSA >2.2 ng/ml. No worrisome adverse events were noted in this cohort.6
Given these early findings demonstrating the clinical utility and safety of 68Ga-PSMA-11 PET/CT in patients with biochemical recurrence, the next step was to prospectively compare this imaging modality to conventional imaging techniques. In 2015, Morigi et al. prospectively evaluated 38 patients who had evidence of a rising PSA following primary therapy (89% radical prostatectomy, 11% radiation therapy). All patients sequentially underwent 18F- fluoromethylcholine PET/CT, diagnostic CT, and then 668Ga-PSMA-11 PET/CT within 30 days. Results were interpreted by two blinded nuclear medicine physicians. Mean PSA level in the cohort was 1.72 ng/mL and 32% of patients had undergone salvage radiotherapy after radical prostatectomy. Overall, 26/37 (68%) patients had a positive scan with 14 having a positive 68Ga-PSMA-11 PET/CT scan only and 11 having both a positive 68Ga-11-PSMA PET/CT and 18F- fluoromethylcholine (1 additional scan was a false positive). Serum PSA level was critical at time of imaging: for PSA <0.5 ng/ml, 50% of 68Ga-PSMA-11 PET/CT scans were positive. For PSAs of 0.5 – 2.0 ng/ml and >2.0 ng/ml, the corresponding positivity rates were 71% and 88%, respectively. Histopathologic confirmation in 9 patients with positive 68Ga-11-PSMA PET/CT scan findings demonstrated evidence of metastatic deposits in all 9 patients. Imaging findings were associated with a major or moderate impact on management in 24 cases (63%). The authors concluded: “In patients with biochemical failure and a low PSA level, 68Ga-PSMA demonstrated a significantly higher detection rate than 18F-fluoromethylcholine and a high overall impact on management.”7
The next question to address was whether PSMA PET/CT was superior to existing, approved imaging modalities in this disease space. Given that 18F- Fluciclovine PET/CT (Axumin® PET/CT) was FDA approved in the biochemical recurrence disease space in 2016, Calais et al. conducted a prospective, single-center (UCLA), open-label, single arm trial of patients with biochemical recurrence following radical prostatectomy who had serum PSA levels of 0.2 – 2.0 ng/ml. All patients underwent 18F-flucicolvine and 68Ga-11-PSMA PET/CT within 15 days of each other, and all scans were interpreted by 3 blinded readers. This trial included 50 patients with a median serum PSA level at enrolment of 0.48 ng/ml. Overall, 68Ga-11-PSMA PET/CT outperformed 18F-flucicolvine with detection rates of 56% (95% CI: 41 – 70%) versus 26% (95% CI: 15- 40%). These differences were most notable for detection of pelvic lymph nodes (30% versus 8%) and any extrapelvic metastatic disease (16% versus 0%):
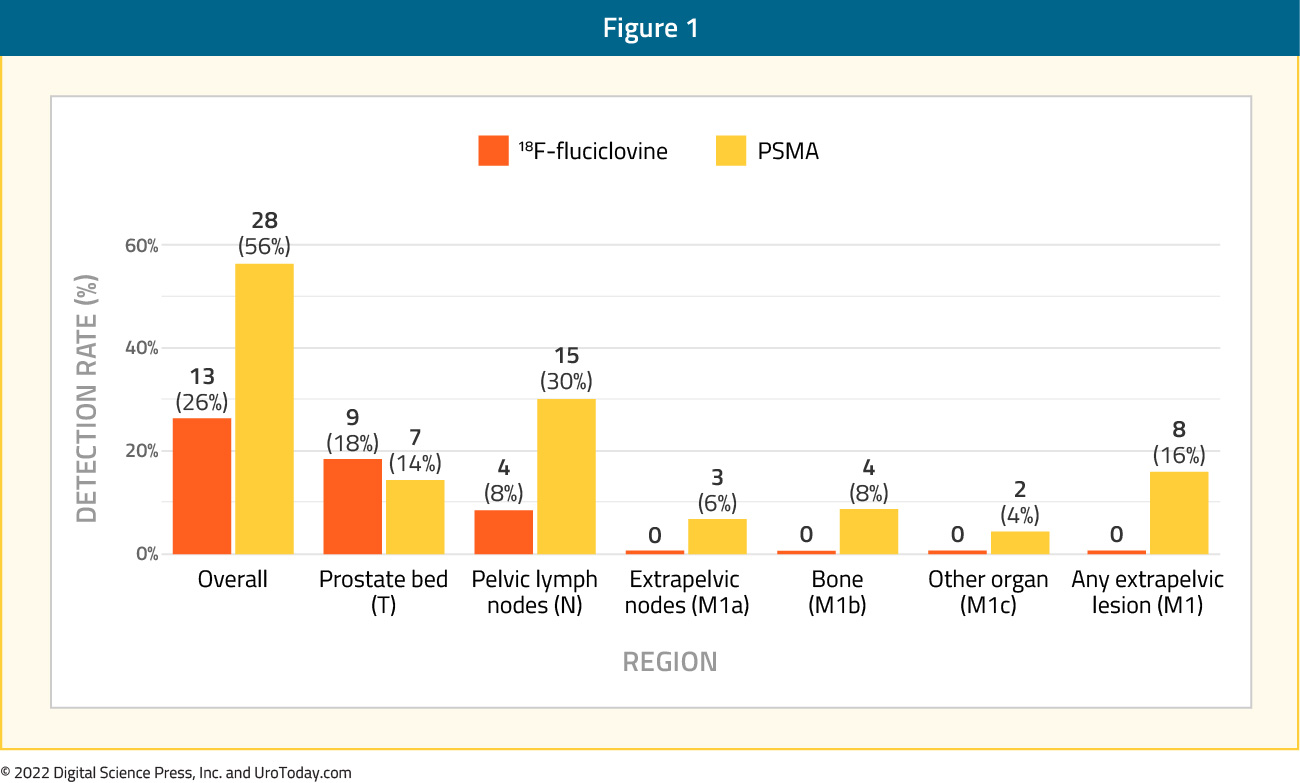
68Ga-11-PSMA PET/CT outperformed 18F-flucicolvine at across all assessed PSA cutoff levels:
- PSA 0.2 – 0.5 ng/ml: 46% versus 27%
- PSA 0.51 – 1.00 ng/ml: 67% versus 28%
- PSA 1.01 – 2.00 ng/ml: 67% versus 17%
Furthermore, inter-reader agreement was superior for 68Ga-11-PSMA PET/CT (κ values ≥0.60) compared to 8F-flucicolvine (κ values ≤0.20).
One of the major limitations to trials of PSMA PET/CT in the biochemical recurrence setting is the lack of consistent histopathologic confirmation of positive imaging findings. Whereas trials in the staging setting were corroborated with histopathologic data from lymph node dissections, this is not practical for patients in the biochemical recurrence space as it is not possible/ethical to perform an excisional biopsy on every PSMA-avid lesion. As such, composite reference standards using a combination of histopathologic and interval clinical and imaging results have been used instead. In this trial, the reference standard included histopathology (n=4), follow-up imaging (n=7), and PSA decreases after PET-directed focal therapy without androgen deprivation therapy (n=4). No false positive findings occurred with either tracer in the 15 patients in whom lesion were verified (i.e. positive predictive value was 100%).8
In the same year, Fendler et al. published the results of a single-arm, multicenter trial conducted at UCLA and UCSF that included 635 patients with biochemical recurrence following prostatectomy (41%), radiation therapy (27`%), or both (32%). Biochemical recurrence was defined as a PSA of ≥0.2 ng/mL 6 weeks post-prostatectomy or a PSA rise ≥2 ng/ml above nadir following radiation therapy. As in the preceding trial by Calais et al., the composite reference standard was the combination of (in descending priority) histopathologic analysis, imaging, and PSA follow-up after local/focal therapy. Of the 635 patients, 42% had composite follow-up at a median duration of 9 months, and 18% had histopathologic follow-up. Median PSA at study entry was 2.1 ng/ml (range: 0.1 – 1,154.0). PET detection rates varied significantly by the PSA at the time of imaging:
- <0.5 ng/ml: 38%
- 0.5 – 0.99 ng/ml: 57%
- 1.0 – 1.99 ng/ml: 84%
- 2.0 – 5.0 ng/ml: 86%
- ≥5.0 ng/ml: 97%
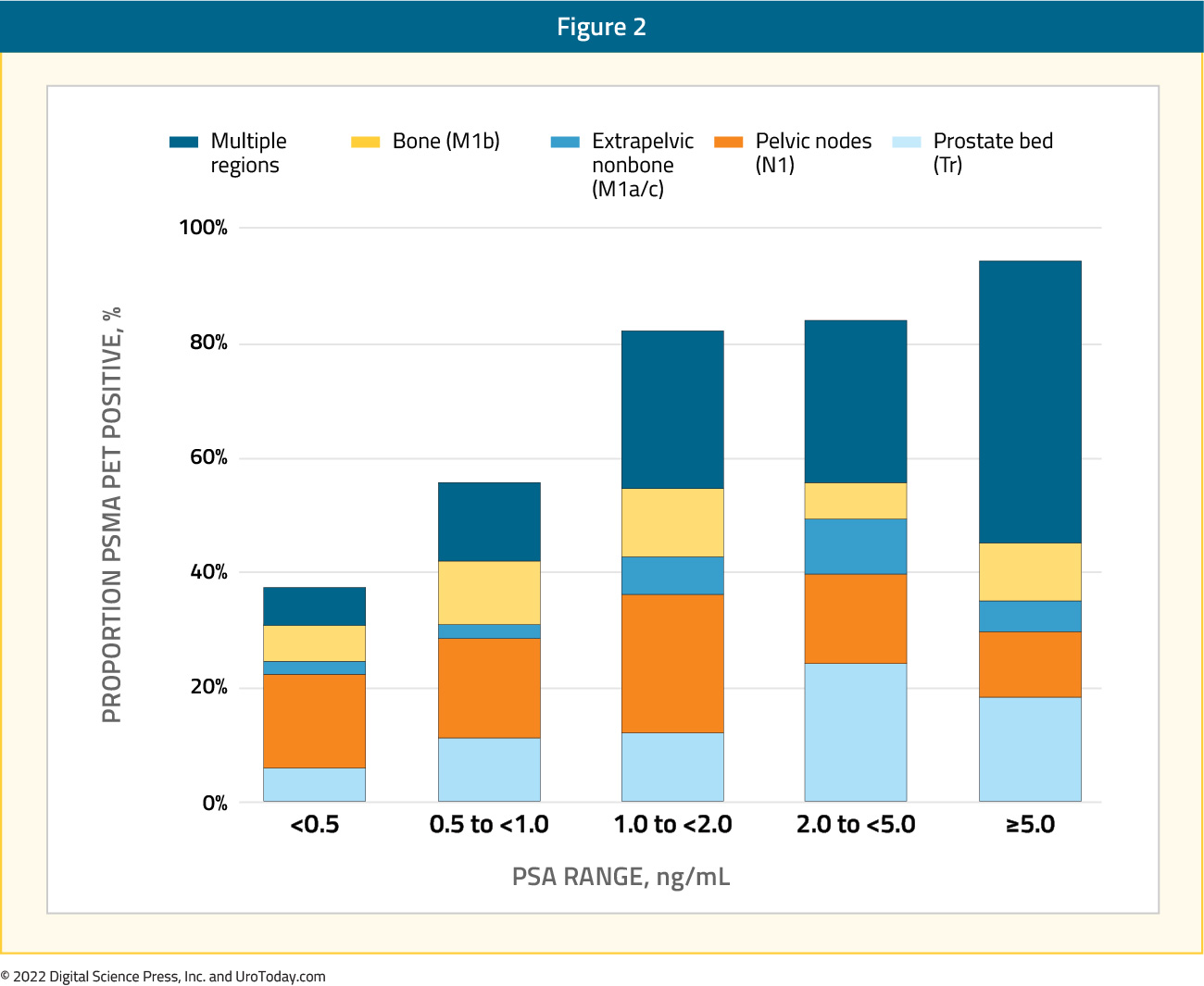
In patients with composite validation (n=217), 92% of PET-positive patents and PET-positive regions were corroborated as positive (i.e. positive predictive value equals 92%). In patients with histopathologic validation (n=87), the positive predictive value was unsurprisingly lower at both the per-patient and per-region basis (84%). This difference in positive predictive value based on the choice of reference standard highlights how the use of a composite reference standard including outcomes other than histopathologic confirmation overestimates the performance of PSMA imaging. Of patients undergoing metastasis-directed focal therapy to PET-positive lesions, any PSA decline was seen in 36 of 39 patients (92%). PSA decline was 50% or higher in 31 (80%) patients (PET true positive). In 10 (26%) of these patients, PSA was undetectable. Inter-reader agreement was κ= 0.65 – 0.78, based on anatomic region.9
Numerous other prospective trials have evaluated the diagnostic performance of 68Ga-PSMA-11 PET/CT in this setting. Caroli et al. prospectively evaluated the performance of 68Ga-PSMA-11 PET/CT CT in 314 patients with biochemical recurrence after prostatectomy or radiation (with or without androgen deprivation therapy). 68Ga-PSMA-11 PET/CT was positive in 197/314 patients (62.7%), with 117/197 lesions (59.4%) limited to the pelvis. Notably, of 88 patients with a negative choline PET/CT, 59/88 (67%) had positive findings on 68Ga-PSMA-11 PET/CT.10
Ceci et al. further evaluated the clinical utility of 68Ga-11-PSMA PET/CT across a number of biochemical failure scenarios: (i) PSA persistence after radical prostatectomy (45/332 patients), (ii) first-time PSA recurrence after radical prostatectomy (149/332), and (iii) PSA increase after salvage or hormonal therapy (138/332). The overall detection rate was 53.6%. The detection rates by subgroup were 64.5%, 45.6%, and 58.7%, respectively. The distribution of relapse location similarly varied by clinical scenario. For patients with persistent PSA following radical prostatectomy, 40% had evidence of locally confined relapse. In contrast, patients with rising PSA after salvage and/or hormonal therapy had locally confined disease in 19.5% per PSMA imaging.11
A meta-analysis of 20 prospective trials in this setting published in June 2022 demonstrated a pooled detection rate of 66.6% for PSMA PET/CT. Analysis of trials including detection rates by PSA levels demonstrated the following positivity rates:
- PSA <0.5 ng/ml: 38.1%
- PSA >2.0 ng/ml: 91.3%
A “major” treatment change (i.e. change in treatment modality) occurred in 42.7% of patients undergoing a PSMA PET/CT, with this figure rising to 63.2% in patients who were PSMA positive.12
18F-DCFPyL-based PSMA PET/CT
The OSPREY trial included a cohort B of patients with presumptive radiological evidence of recurrent or metastatic prostate cancer on conventional imaging and in whom lesions were considered amenable to biopsy. This cohort included 117 men, 63% of whom had previously received hormonal therapy. At time of study entry, conventional imaging demonstrated evidence of locoregional disease in 28% of patients and distant disease in the remaining 72%. The median PSA level was >2 ng/ml in 72.6% of patients.
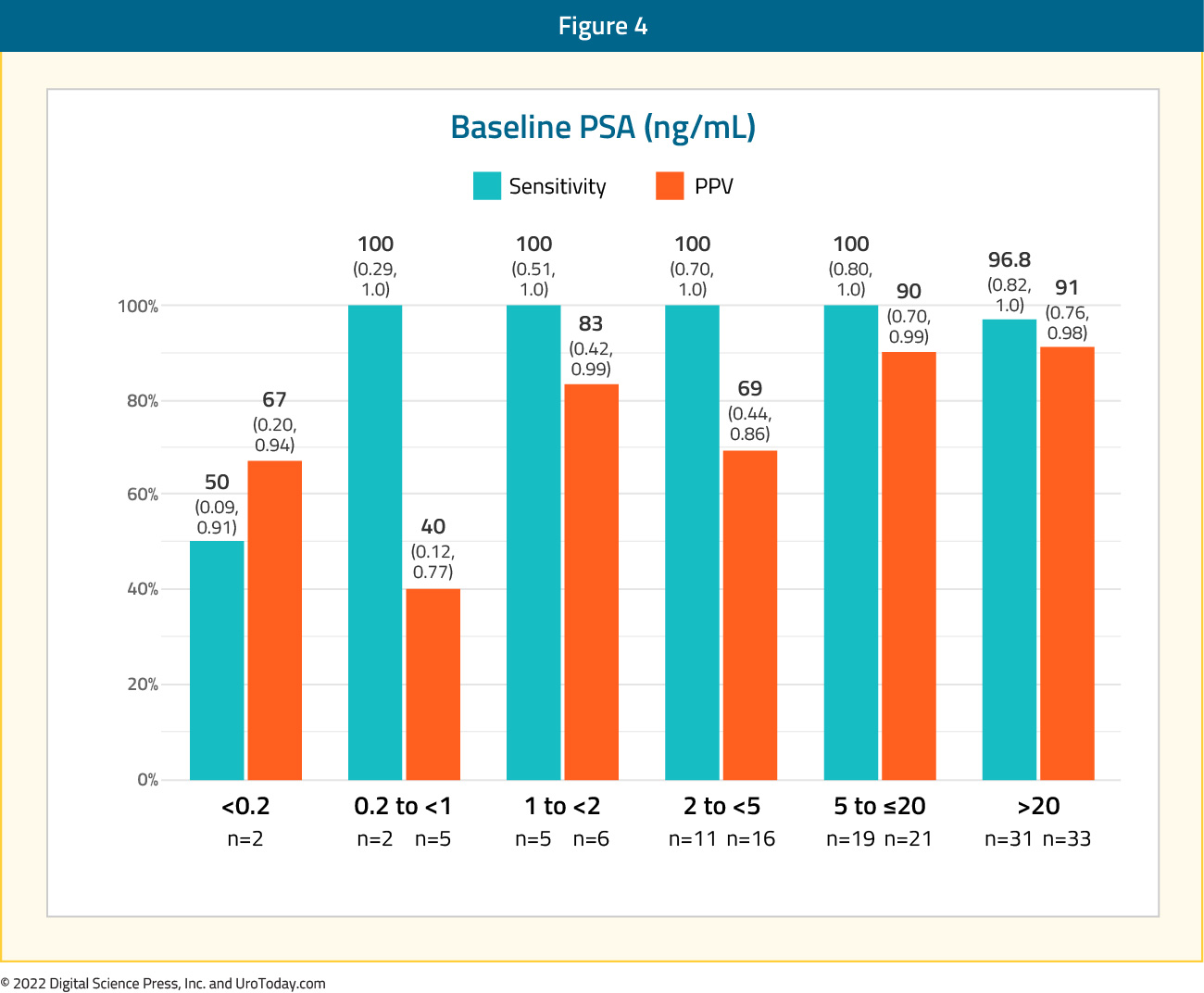
The sensitivity and positive predictive value of 18F-DCFPyL-PET/CT in this setting was 95.8% (95% CI: 87.8 - 99.0%) and 81.9% (95% CI: 73.7- 90.2%), respectively. As seen below, this test performed similarly regardless of site of metastasis.

The sensitivity of 18F-DCFPyL-PET/CT was largely dependent on PSA levels with sensitivity of 50% for PSA <0.2 ng/ml, and nearly 100% for all other levels.
Published concurrently in July of 2021, the CONDOR trial is a prospective, multicenter, open-label phase III trial that evaluated 18F-DCFPyL-PET/CT in men with biochemical recurrence following prostatectomy or radiotherapy, defined as PSA ≥0.2 ng/ml after radical prostatectomy and PSA≥2 ng/ml post-radiation. Whereas patients in OSPREY Cohort B had evidence of locoregional or distant disease on conventional imaging, all patients in CONDOR had negative or equivocal conventional imaging (including 18F-fluciclovine or 11C-choline PET, CT, MRI, and/or whole-body bone scintigraphy) within 60 days prior to 18F-DCFPyL-PET/CT. Patients were excluded if they had received gamma-emitting radioisotope within five physical half-lives prior to 18F-DCFPyL injection, and androgen deprivation therapy within 3 months of imaging, or investigational therapy for prostate cancer within 60 days of imaging.
Following FDA recommendations, the primary endpoint chosen was correct localization rate (CLR), defined as the positive predictive value with an additional requirement of anatomic lesion co-localization between 18F-DCFPyL-PET/CT and a composite standard of truth. The FDA a priori suggested a meaningful CLR was 20% at PSA < 0.5 ng/mL Because of the anticipated lack of a lesion amenable for histologic verification in most patients, a composite standard of truth (in order of priority) was defined as: (1) evaluable histopathology results from prostatectomy, salvage pelvic lymph node dissection or targeted biopsy; (2) correlative follow-up imaging findings using 18F-fluciclovine or 11C-choline PET, or focused MRI or CT; or (3) if neither of the above was available or informative, confirmed PSA response up to 9 months post-radiation initiation (without concomitant ADT) of all PET-positive foci. PSA response was defined as PSA decline by ≥50% from baseline that was confirmed on repeat measurement within 4 weeks, based on central laboratory results.
This trial included 208 patients with a median PSA level of 0.8 ng/ml (range: 0.2 – 98.4). Patients had received radical prostatectomy alone in 49.5% of cases, radiation alone in 14.9%, and both modalities in 35.6% of cases. 18F-DCFPyL-PET/CT was positive in 59.1-65.9% of patients as assessed by the three independent blinded central readers The CLR ranged between 84.8% and 87.0% among the three readers.
There was a clear association between increasing baseline PSA level and both improved detection and correct localization rates as demonstrated below:
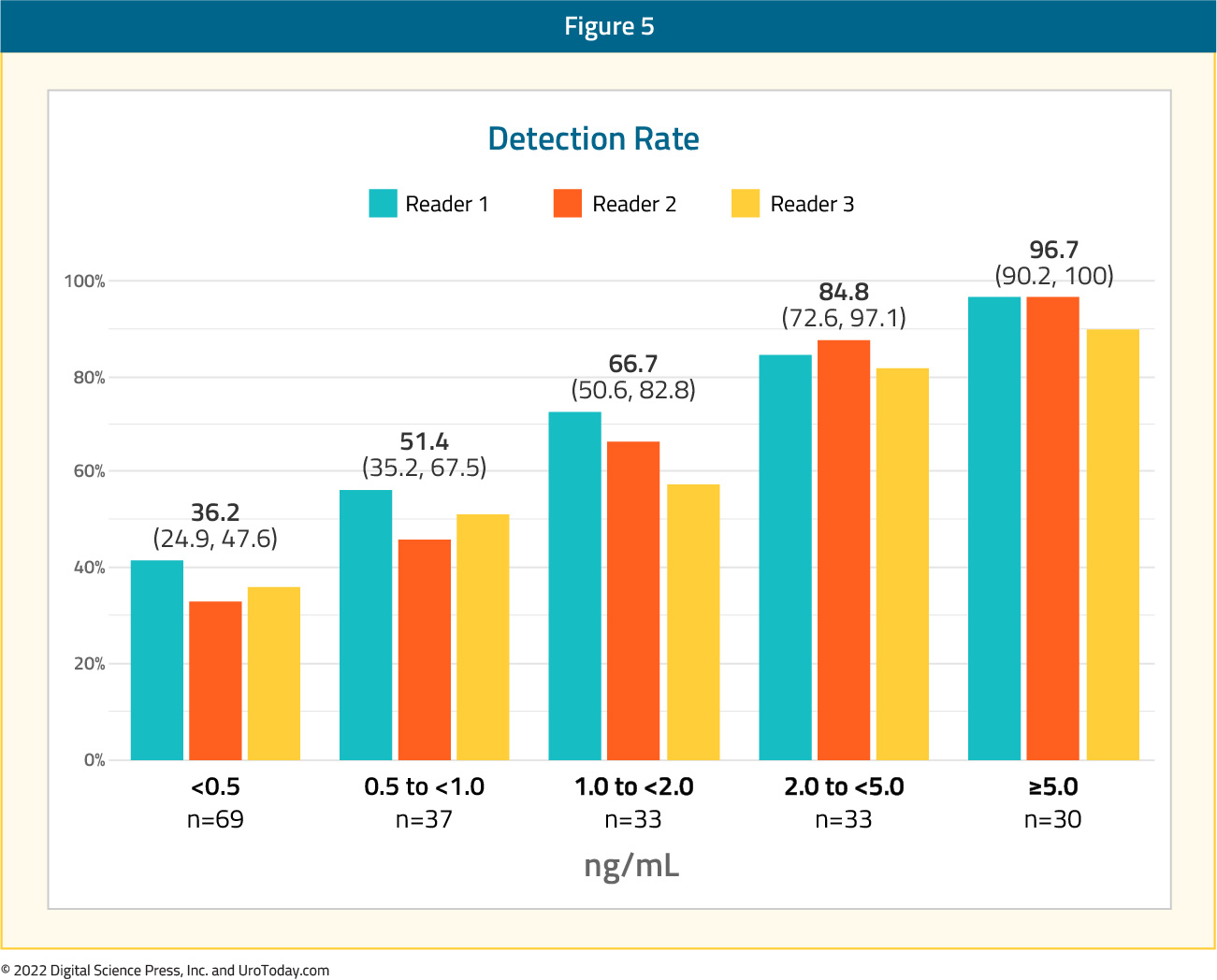

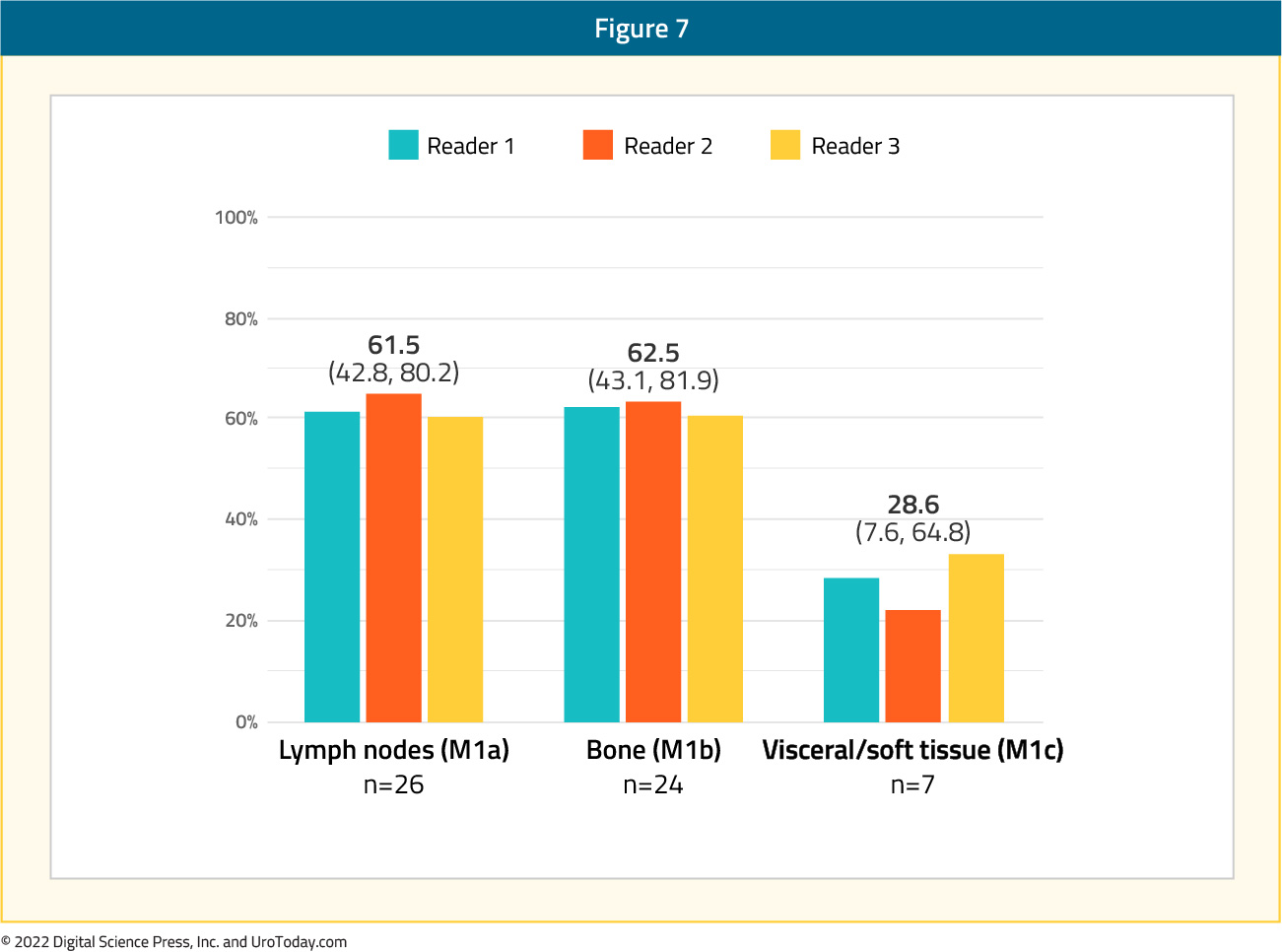
Next, the authors examined the correct localization rate by anatomic region with values of 79.5% for the prostatic fossa, 70.9% for pelvic lymph nodes, and 67.4% for extra-pelvic (M1) disease. Within the M1 subgroup, the correct localization rates were lowest for visceral/soft tissue (M1c) disease (28.6%) compared to lymph nodes (M1a) at 61.5% and bone (M1b) at 62.5%. Inter-reader agreement had a concordance of 75% and Fleiss’ kappa of 0.65 (95%CI: 0.58, 0.73).
The rationale for performing PSMA PET/CT imaging in this disease space is that potential findings from this modality may provide new information that influence treatment decisions. In the CONDOR trial, 131 (64%) patients had a change in intended disease management plan. Of these 131 patients, 103 (79%) had positive 18F-DCFPyL-PET/CT findings, and conversely, of the 144 patients with a positive 18F-DCFPyL-PET/CT, 103 (72%) had a recommended change in management. The most frequent changes to treatment management plans after the 18F-DCFPyL-PET/CT imaging results included salvage local therapy that was either supplemented or replaced by systemic therapy (n = 58; 28.3%), observation to initiating therapy (n = 49; 23.9%), systemic therapy to salvage local therapy (n = 43; 21.0%), and planned treatment to observation (n = 9; 4.4%).13
But do these changes in treatment decisions have a meaningful impact on long-term patient outcomes? Given the current evidence, the answer to this question remains unknown. Ong et al. conducted a multicenter prospective trial across multiple Australian centers of 100 men with biochemical recurrence, following a radical prostatectomy and/or radiation therapy, who received a 68Ga-PSMA-11 PET/CT. Median PSA was 0.82 ng/ml. With a median follow-up of three years, 25% of patients had treatment progression (i.e. the addition or change of any treatment modalities such as androgen deprivation therapy, radiation therapy or chemotherapy). There appeared to be an association between findings on 68Ga-11-PSMA PET/CT and treatment progression: in men with a positive 68Ga-PSMA-11 PET/CT, 30.1% of patients had treatment progression compared to 15.1% of patients with a negative 68Ga-PSMA-11 PET/CT. Of the patients who had no treatment progression, 2/63 (3.2%) had a rise in PSA or disease seen on imaging but no addition or change in treatment.14 The corresponding outcomes in patients with treatment progression, as of yet, have not been reported.
Another Australian prospective multicenter trial attempted to shed light on this question. Emmett et al. prospectively enrolled 260 men with biochemical recurrence following radical prostatectomy who received a 68Ga-PSMA-11 PET/CT with a median PSA of 0.26 ng/ml (IQR: 0.15-0.59 ng/ml). Positive 68Ga-PSMA-11 PET/CT results were seen in 170/260 patients (65.4%), with 21.5% having disease confined to the prostate, 26.2% having pelvic nodal involvement, and 17.7% having distant nodal, bone, or visceral involvement. Among the 260 patients, 186 (71.5%) received salvage radiation therapy with 25% of these patients receiving concurrent ADT. PSMA-positive findings were associated with increased likelihood of receiving wider field radiation therapy and hormonal therapy. Amongst patients receiving salvage radiation, 3-year freedom from progression (i.e., PSA rise of no more than 0.2 ng/ml above nadir after salvage radiation without additional treatment) was highest in patients with negative PSMA PET/CT findings (82.5%), followed by patients with PSMA PET/CT-avid disease confined to the prostatic fossa (79%), pelvic nodes (55%), distant lymph nodes (25%) and viscera or bones (21%).15
The findings from these two trials shed light on the short-term prognostic implications of PSMA 68Ga-PSMA-11 PET/CT findings, but as of yet, it remains unknown whether management changes secondary to PSMA PET/CT findings truly influence long-term patient clinical outcomes. A meta-analysis of 34 studies (many retrospective in nature), reported a pooled proportion of change in management after PSMA PET/CT of 56.4% (95% CI: 48.0 - 63.9%). A decrease in serum PSA was reported in 72.4% of men (95% CI: 63.4 – 81.5%), and a complete biochemical response in 23.3% (95% CI: 14.6 – 32.0%) at a median follow-up of 8.1 and 11 months, respectively. With a median follow up of 20 months, the pooled biochemical recurrence-free rate was 60.2% (95% CI: 49.1 – 71.4%).16
Conclusions
PSMA PET/CT is recommended by the NCCN guidelines for men with biochemical recurrence after definitive primary local therapy. Multiple studies using 68Ga-PSMA-11 PET/CT and the key CONDOR trial assessing 18F-DCFPyL-PET/CT led to these recommendations, also resulting in a change in management in approximately 50 to 65% of patients. Further longitudinal studies are required to assess the true impact of these changes in management, however, regardless, the utilization of PSMA PET/CT has appropriately intensified (and deintensified) salvage treatment for men with biochemical recurrence.
Published October, 2022
Written by:
- Rashid Sayyid, MD MSc, University of Toronto, Toronto, ON
- Zachary Klaassen, MD MSc, Medical College of Georgia, Augusta, Georgia, USA


