Introduction
Bladder cancer remains the sixth most commonly diagnosed cancer in the United States, with an estimate of 82,290 incident cases in 2023.1 At diagnosis, approximately 75% of patients present with non-muscle invasive disease, with significant clinical heterogeneity observed within this disease group.2,3 Patients with initial low-grade Ta disease (i.e., confined to the mucosal lining) represent a unique patient cohort given their favorable long-term oncologic outcomes, given that they are more likely to recur than progress to life-threatening disease.4
However, these patients require repeated, long-term surveillance with frequent ablative procedures, often including transurethral resection of the bladder tumor (TURBT). This has important implications from a health services utilization perspective, significantly increases the economic/financial burden of treating bladder cancer, and, importantly, has adverse effects on patient quality of life.5 Given that the risk of disease progression is low in low-grade Ta patients, most of the efforts in this disease space have focused on reducing the rate of disease recurrence. Current adjuvant options with proven efficacy for reducing recurrence rates include post-operative continuous bladder irrigation,6 a single, immediate, intravesical instillation of chemotherapy within 24 hours post-operatively,7-9 device-assisted intravesical chemotherapy,10,11 and intravesical bacillus Calmette- Guérin (BCG),12-15 classically reserved for the intermediate-risk subgroup of low-grade Ta patients.
Low-grade, low-risk Ta NMIBC is often managed with only periodic cystoscopy, however, the risk of recurrence leads to patient “follow-up fatigue” and utilization of precious healthcare resources. Primary chemoablation, which refers to the direct destruction of tumor cells using intravesical chemotherapeutic agents, has long been evaluated as a potential treatment approach to safely reduce the requirement for repeat TURBTs, with mixed results. However, over the last few years, we have witnessed the emergence of UGN-102, an investigational agent consisting of mitomycin plus a proprietary reverse thermal hydrogel (UroGen® Pharma) which has demonstrated encouraging results for the primary, non-surgical treatment of low-grade, intermediate-risk NMIBC. In this Center of Excellence Article, we will discuss the current landscape of managing low-grade Ta NMIBC, including primary intravesical chemoablation with a specific focus on the recently published Optima II and ATLAS trials evaluating UGN-102.
Primary Chemoablation Prior to UGN-102
Epirubicin
A randomized trial of primary chemoablation using intravesical epirubicin instillations was initially reported in 1994. Eighty-one patients with superficial bladder cancer underwent endoscopic resection of all but one papillary ‘marker’ tumor and were subsequently randomized to receive intravesical epirubicin at a concentration of either 1 mg/ml (n = 40) or 2 mg/ml (n = 41) in 50 ml of saline for one hour. At the three months cystoscopic assessment, a complete response, defined as no visible or microscopic bladder cancer, was observed in 46% of patients (95% CI 35 to 57%). Chemical cystitis and bladder irritability were the most frequent local side effects, occurring in 15% of patients.16
Mitomycin
The feasibility of intravesical mitomycin-C instillations for the primary chemoablation of low-risk non-muscle invasive bladder cancer (NMIBC) was recently assessed in the phase II CALIBER trial. Eighty-two patients with recurrent, low-risk NMIBC were randomized 2:1 to treatment with four once-weekly mitomycin-C intravesical instillations or TURBT. A complete response at 3 months was observed in 37% of mitomycin-C treated patients, compared to 81% in the TURBT arm.17

The DaBlaCa-13 study evaluated short-term, intensive chemoresection with intravesical mitomycin-C (40 mg/ml) administered three times weekly for two weeks (n = 58) versus TURBT with six weekly adjuvant instillations (n = 61). A complete tumor response at 4 weeks was observed in 57% of patients in the mitomycin-C arm. Importantly, there were no significant difference in response rates between patients with low- or high-grade tumors. There was one grade 3 adverse event (cystitis) reported in the mitomycin-C arm.18

Primary Chemoablation with UGN-102
UGN-102 is an investigational drug consisting of mitomycin-C combined with a proprietary reverse thermal hydrogel used to reconstitute mitomycin prior to instillation. The reverse thermal properties of UGN-102 allow for the local administration of mitomycin as a liquid in a cooled state, with subsequent conversion to a semi-solid gel depot at body temperature post-instillation. The gel slowly disintegrates and is subsequently eliminated by voiding, allowing for the sustained release of mitomycin over a period of four to six hours. The prolonged exposure of tumor cells to mitomycin is hypothesized to improve chemoablation compared with aqueous preparations of the drug. An analogous agent exists in the upper tract disease space with UGN-101 (Jelmyto®) that has demonstrated efficacy in the OLYMPUS trial and has since been FDA-approved for adult patients with low-grade upper tract urothelial cancer.19,20
The efficacy and safety of UGN-102 has recently been evaluated in two prospective trials: Optima II and ATLAS.21,22
Optima II
Optima II was a prospective, phase 2b, open-label, single-arm, multicenter trial of UGN-102 in patients with biopsy-proven low-grade intermediate-risk NMIBC. Eligible patients were diagnosed via cold cup biopsy, with visible tumor left in situ, and had a voiding cytology negative for high-grade disease. Intermediate-risk disease was defined as having one or two of the following three risk factors:
- Presence of multiple tumors
- Solitary tumor >3 cm
- Recurrence (≥1 occurrence of low-grade NMIBC within one year of the qualifying diagnosis)
Eligible patients received six once-weekly intravesical instillations of UGN-102 (75 mg mitomycin in 56 ml admixture with reverse thermal hydrogel to equal 1.33 mg/ml). The primary outcome was complete response, defined by a negative cystoscopy (biopsy of visualized lesions only) and a negative voided urine cytology, and was evaluated three months following treatment initiation.

This trial included 63 patients all of whom received ≥1 UGN-102 instillation. All six instillations were tolerated by 57 (90%) patients, with the remaining six discontinuing treatment due to adverse events. The median patient age was 68 years (range: 33 – 96), 49 (78%) had recurrent low-grade disease, and 28 (44%) had a previous episode within one year of the latest diagnosis. Of note, 82% of patients had multifocal disease and 28% had tumors >3 cm.
A complete response at three months was observed in 41 (65%) patients. Of these 41 patients, 39 (95%), 30 (73%), and 25 (61%) remained recurrence-free at six, nine, and 12 months following treatment initiation, respectively. The median duration of complete response was not estimable.
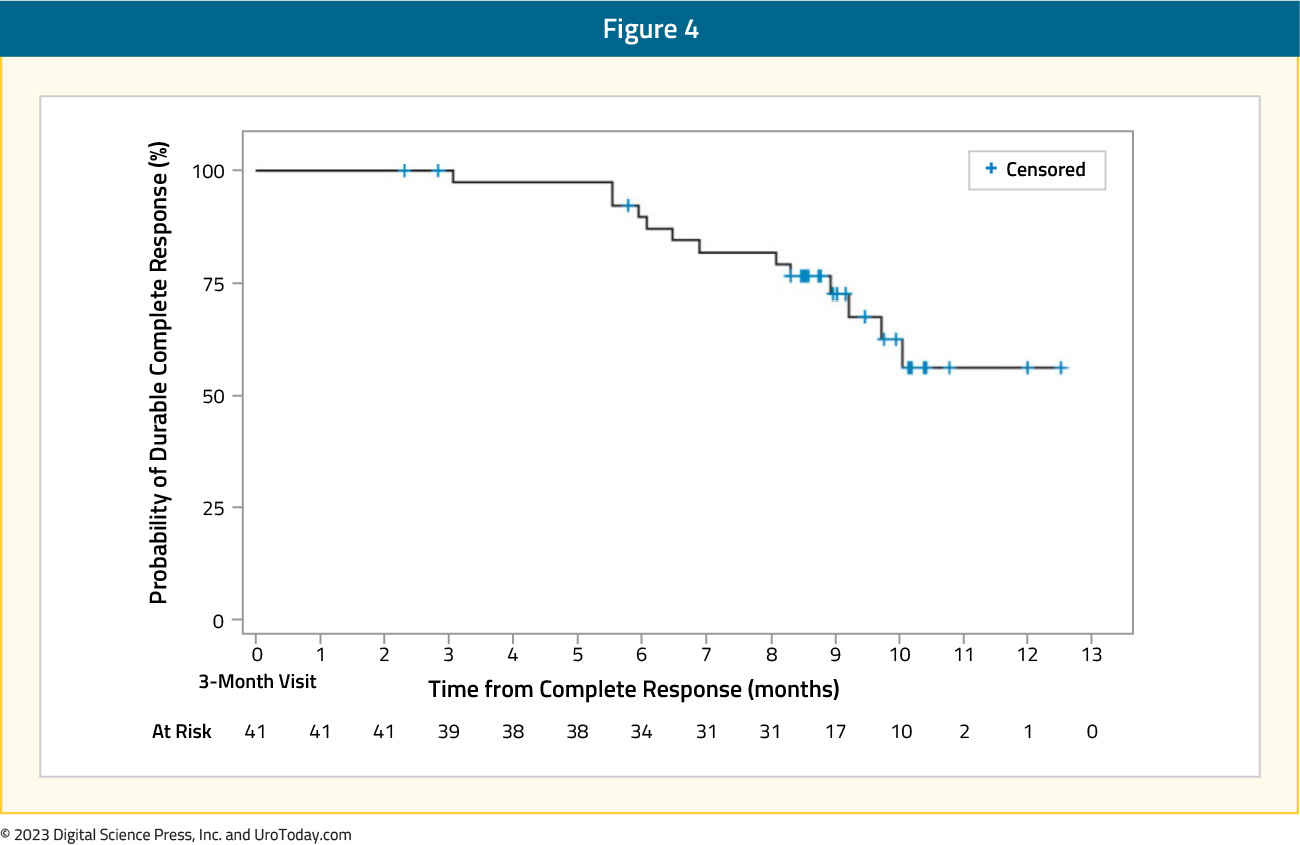
Among the remaining 22 patients who did not achieve a complete response, 20 showed evidence of disease persistence or worsening, including five who had high-grade papillary disease and/or carcinoma in situ, suggesting under-grading at the time of diagnosis.
Treatment-related adverse events of any grade were observed in 63% of patients. The most frequently reported treatment-related adverse events were dysuria (41%), urinary frequency (21%), hematuria (16%), micturition urgency and urinary tract infection (14%), and fatigue (11%). Grade 3 or worse events occurred in 8% of patients, with the most common being hematuria and penile edema (1.6% each).21
ATLAS
Following the promising results of the single arm, phase two Optima II trial, UGN-102 was assessed in ATLAS, a prospective, phase 3, randomized, open-label trial that randomized patients with low-grade, intermediate-risk NMIBC in a 1:1 fashion to either UGN-102 +/- TURBT or TURBT alone (no adjuvant therapy). Eligibility criteria were similar to those of Optima II, and patients in the UGN-102 arm of ATLAS also received six weekly intravesical instillations. Patients in this arm with a complete response at 3 months received no further treatment, whereas those with residual low-grade disease in either treatment arm underwent a TURBT for any remaining lesions. The primary study endpoint was disease-free survival. Of note, following the enrollment of 282 of the planned 632 study participants, further study enrollment was suspended early by the sponsor in order to pursue an alternative development strategy for UGN-102 for the treatment of bladder cancer. Patients who had consented at the time of trial termination were permitted to continue, but follow-up was discontinued once the last patient had been followed for 15 months after treatment initiation.

Among the 282 patients, 142 and 140 were randomized to UGN-102 +/- TURBT and primary TURBT, respectively. The median patient age was 68 years, and approximately 42% of patients had recurrent disease, with 29% having recurrence within the year prior to study enrollment. Multifocal disease was present in 63% of patients, and 45% had evidence of tumors >3 cm. Of the 142 patients randomized to UGN-102, 132 (96%) completed all six instillations.
At the first disease assessment at 3 months post-treatment initiation, a complete response was observed in:
- UGN-102 +/- TURBT arm: 65% (95% CI: 56 – 73%)
- TURBT alone arm: 64% (95% CI: 55 – 72%)
Disease progression at the 3-month assessment was noted in 12 UGN-102 patients and 9 TURBT patients. Among patients with residual low-grade disease at the 3-month assessment, 24/26 treated with UGN-102 and 18/22 treated with primary TURBT underwent a TURBT. Disease-free survival 15 months after randomization was estimated to be 72% for patients in the UGN-102 ± TURBT arm and 50% for patients in the TURBT monotherapy arm, with a hazard ratio of 0.45:

Among patients achieving a complete response at the 3-month assessment, the estimated 12-month maintained response was 80% following induction treatment with UGN-102 and 68% following primary TURBT, with a hazard ratio of 0.46:

Treatment-related adverse events occurred more commonly in the UGN-102 arm (39% versus 11%), with serious events in 8.7% and 5.3%, respectively (none related to treatment per investigator assessment). The most frequent adverse events in the UGN-102 +/- TURBT arm were dysuria (30%), micturition urgency (18%), nocturia (18%), and pollakiuria (16%). Patient-reported symptoms, functioning, and quality of life (assessed via the EORTC-QLQ-NMIBC24) were either improved or not worsened in both arms.22
Conclusions
Primary chemoablation with UGN-102 has emerged as an effective treatment option for patients with either primary or recurrent low-grade, intermediate-risk NMIBC. While we await potential regulatory approval for this investigational drug in the upcoming future, this agent may prove to be an attractive treatment modality for patients wishing to minimize the frequency of repeat TURBTs due to quality of life and financial concerns or who have significant medical comorbidities precluding operative interventions. The results of the phase 3 ENVISION (NCT05243550) open label, single-arm trial assessing the safety and efficacy of UGN-102 in intermediate risk recurrent NMIBC will be much anticipated and hopefully continue to push the envelope for primary chemoablation of NMIBC.


