Introduction
Classical theories of metastasis have followed ‘the seed and soil’ hypothesis, the Halstedian model, which proposes an orderly spread of disease from local to distant sites, with the presumption that cancer is an inherently systemic process even in the earliest cases. More contemporary spectrum theories now suggest that the propensity for distant spread exists along a ‘metastatic continuum’. Tumors with limited metastatic potential (i.e., oligometastases) represent a unique subset along this spectrum that could be potentially cured with local ablative therapy (i.e., metastasis-directed therapy [MDT]). This concept is not unique to prostate cancer and has been evaluated in other disease sites including non-small cell lung, colorectal, esophageal, and breast cancers.1
The majority of the evidence for MDT in the prostate cancer space, to date, has been for patients with recurrent, oligometastatic hormone sensitive prostate cancer. MDT has been evaluated both in lieu of, to avoid/delay the use of systemic therapy, and in combination with systemic therapy to potentially improve efficacy outcomes. In this Center of Excellence article, we discuss the evidence and practical applications for MDT in the recurrent oligometastatic hormone sensitive setting.
Trials of MDT versus Observation/Surveillance for Recurrent Oligometastatic Prostate Cancer
To date, three prospective phase II trials have compared MDT to observation for patients with recurrent oligometastatic hormone sensitive prostate cancer.
STOMPThe STOMP trial was a multicenter, randomized phase II trial that prospectively evaluated the effects of MDT for eugonadal men with evidence of oligometastatic disease on choline PET/CT (up to three extracranial sites) who had received prior treatment with curative intent and had evidence of biochemical recurrence. Between 2012 and 2015, 62 patients were randomized 1:1 to either surveillance or MDT, consisting of stereotactic body radiotherapy (SBRT) or metastasectomy. The primary endpoint was time to initiation of ADT (i.e., ADT-free survival). ADT was initiated for symptoms, progression beyond three metastases, or local progression of known metastatic disease
After a median follow up of 5.3 years, the five-year ADT-free survival was 8% in the surveillance arm compared to 34% for the MDT group (HR: 0.57, 95% CI: 0.38–0.84, log-rank p = 0.06). No differences were seen between groups when stratified by nodal versus non-nodal metastases:
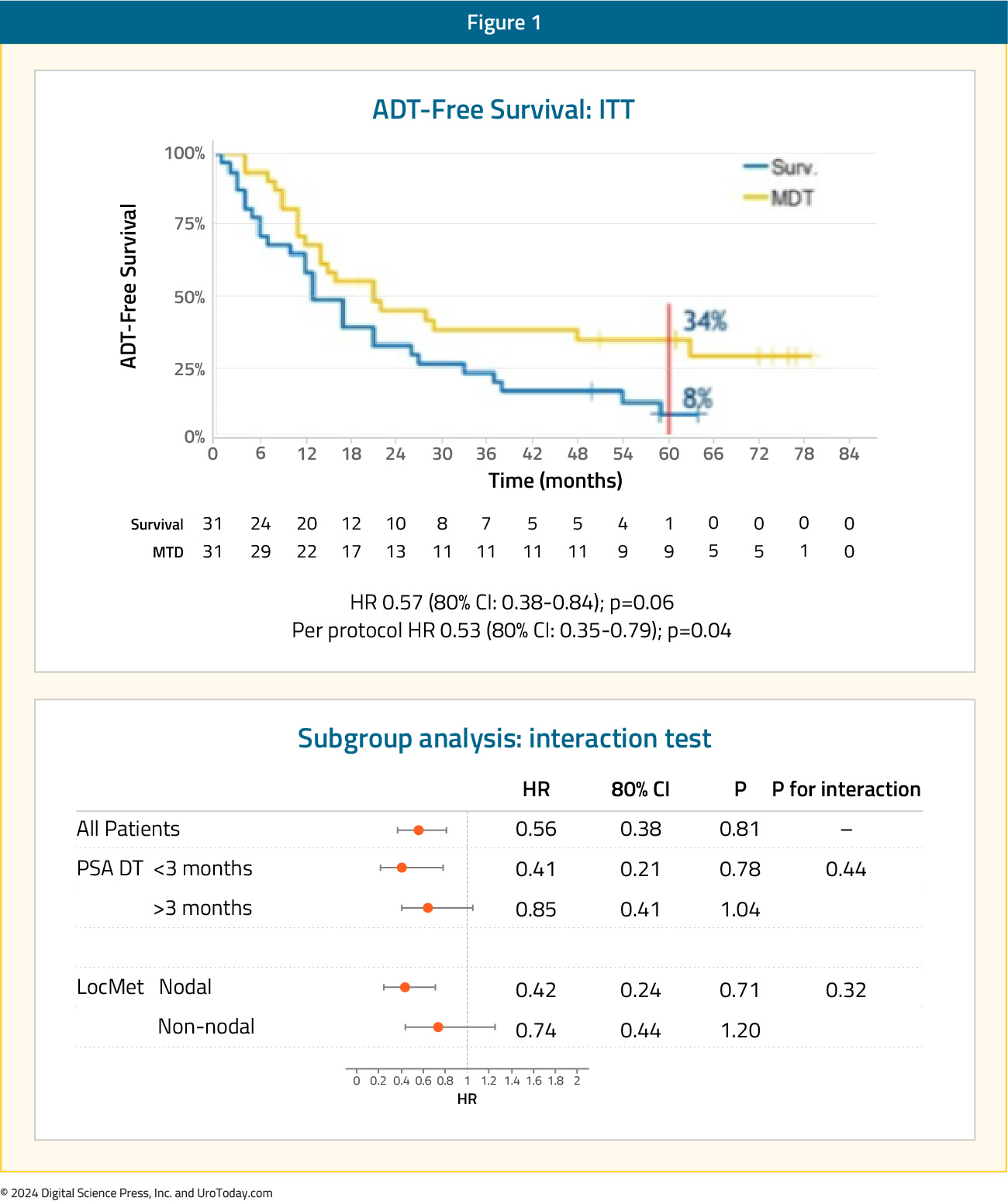
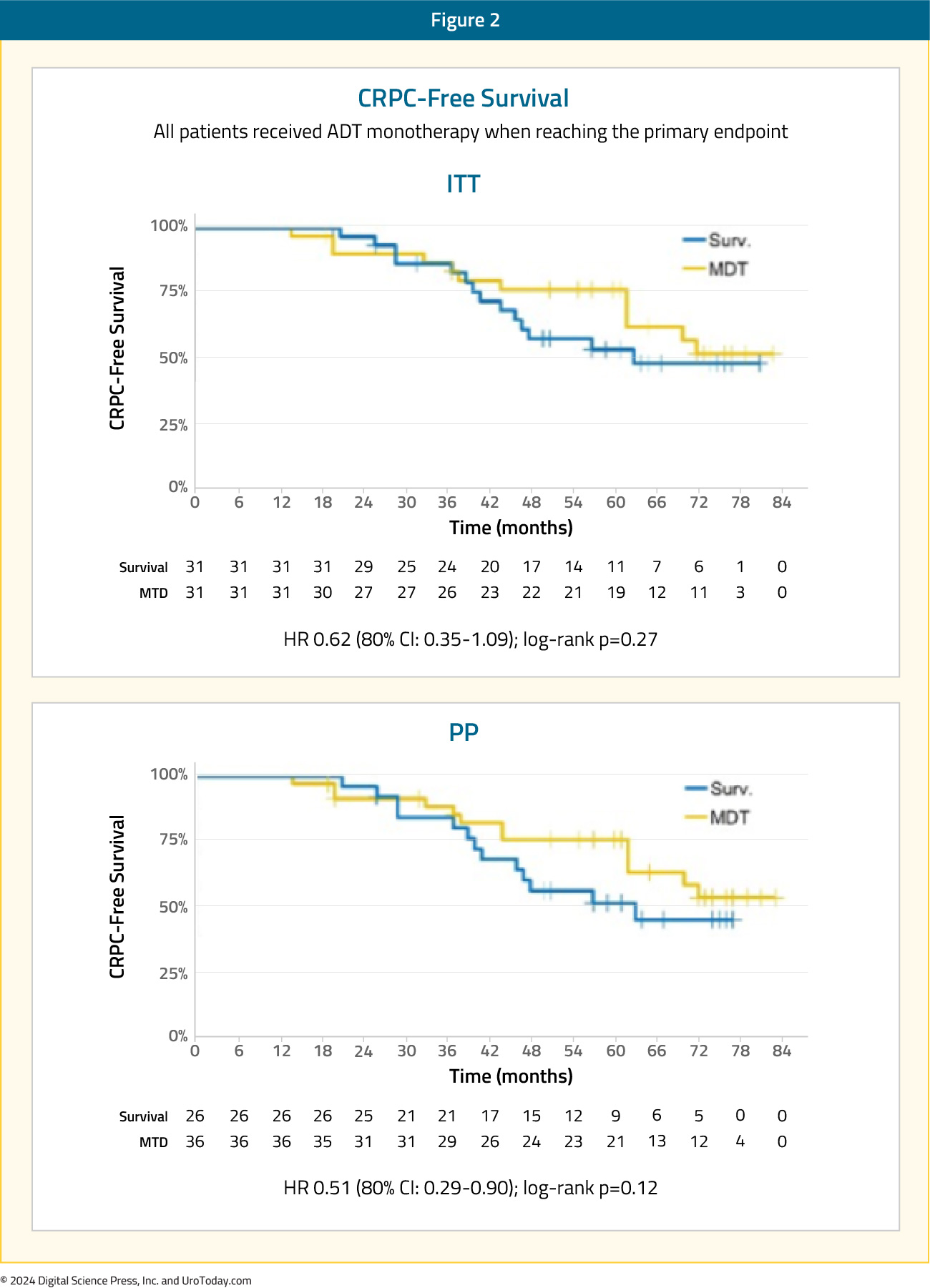
The secondary endpoint of 5-year CRPC-free survival was 53% in subjects under surveillance and 76% in those receiving MDT (HR 0.62, 80% CI: 0.35-1.09):2
The ORIOLE trial was a randomized phase II trial of men with recurrent oligometastatic hormone-sensitive prostate cancer (up to three sites). Between 2016 and 2018, 80 men were screened, of which 54 men had 1 to 3 metastases detectable by conventional imaging and had not received ADT within 6 months of enrollment or 3 or more years total. These 54 men were randomized in a 2:1 fashion to receive SBRT or observation. The primary outcome was disease progression at 6 months, defined by a serum PSA increase, radiographic progression on conventional imaging, symptomatic progression, ADT initiation for any reason, or death.
After a median follow-up of 19 months, disease progression at six months occurred in 19% of patients in the SBRT arm versus 61% of patients in the observation arm (p = 0.005). Patients in the SBRT treatment arm had superior median progression-free survival rates (median: not reached versus 5.8 months; HR: 0.30; 95% CI: 0.11–0.81; p = 0.002):
Secondary to the blinding of the investigative team to the PSMA-targeted PET data during treatment planning, 16 of 36 men treated with SBRT had baseline PET-avid lesions that were not included in the treatment fields. The proportion of men with no untreated lesions with progression at 6 months was 1 of 19 (5%) compared with 6 of 16 (38%) for those with any untreated lesions (p = 0.03). The median progression free survival was unreached among men with no untreated lesions vs 11.8 months among participants with any untreated lesions (HR, 0.26; 95% CI, 0.09-0.76; p = 0.006):3
As such, this was among the first data to suggest the importance of treating all PSMA PET-visible lesions for maximal oncologic benefit in the oligometastatic mHSPC space.
ORIOLE + STOMP: Pooled DataIn 2022, pooled data from the ORIOLE and STOMP trials demonstrated that MDT improves progression free survival from 5.9 months to 11.9 months (HR: 0.44, p<0.001), however without any significant improvements seen in radiographic progression-free survival, time to castration-resistant disease, or overall survival:4

SABR-COMET was a randomized, open-label phase II study of patients with oligometastatic disease (up to five sites) between February 2012 and August 2016. This trial was not restricted to patients with prostate cancer and also included lung, breast, and colorectal cancer patients. Of the 99 patients in this trial, 18 (18.2%) had prostate cancer. After stratifying by the number of metastases (1–3 versus 4–5), patients were randomized in a 1:2 fashion to receive either palliative standard of care alone or standard of care plus SBRT.
At a median follow-up of 5.7 years, the primary outcome of overall survival was superior for SBRT-treated patients. The 8-year overall survival rates were 27% and 14% in the intervention and control arms, respectively (HR: 0.50, 95% CI: 0.30–0.84, p = 0.008). The 8-year progression-free survival estimates were 21% and 0%, respectively (HR: 0.45, 95% CI: 0.28–0.72, p < 0.001):

The rates of grade ≥2 acute or late toxic effects were 30% versus 9% (p = 0.019), and the FACT-G quality of life scores declined over time in both arms, but with no differences in quality-of-life scores between the study arms.5
Combination of MDT + Systemic Therapy for Recurrent Oligometastatic Prostate Cancer
EXTENDThe EXTEND trial was a single center, phase II randomized controlled trial of 87 oligorecurrent men, mostly with mHSPC (>90%), who were randomized 1:1 to intermittent hormone therapy +/- MDT (definitive radiation therapy to all sites of disease). All patients had ≤5 metastases, as defined by conventional imaging (75%) or fluciclovine PET/CT (25%). A planned break in hormone therapy occurred 6 months after enrollment, after which hormone therapy was withheld until progression. At a median follow-up of 22 months, progression free survival was improved in the combined therapy arm (HR: 0.25, 95% CI: 0.12 – 0.55, p < 0.001). Significantly, ‘eugonadal’ progression free survival was also improved with this combination approach (HR: 0.32, p = 0.03):6
SATURN is a phase II trial of 28 men with oligorecurrent extra-pelvic metastases on PSMA-PET/CT following initial treatment with radical prostatectomy. Patients were treated with 6 months of ‘androgen annihilation therapy’, defined as leuprolide + abiraterone acetate/prednisone + apalutamide. After the 1st month of this systemic therapy, patients received SBRT to all metastases with or without radiotherapy directed to the prostate bed and pelvic lymph nodes. Results of the primary endpoint, the percentage of patients who maintained PSA <0.05 ng/mL six months after testosterone recovery to ≥150 ng/dL, were presented at ASCO GU 2024. Overall, 50% of patients maintained a PSA <0.05 ng/mL six months after testosterone recovery. After a median follow-up of 20 months, the median progression free survival was 19.3 months. Moreover, 81% of patients recovered eugonadal testosterone levels, at a median of 9.4 months from the start of systemic therapy, and the median eugonadal progression free survival was 11.4 months. Grade 3 adverse events related to androgen therapy were observed in 21% of patients, and SBRT had 7.7% grade 2 and no grade 3 toxicity.9
Key Ongoing Trials of MDT + Systemic TherapyThere are several important ongoing trials combining systemic therapy with the site specific control offered by MDT. The ADOPT trial is an ongoing phase III trial that is randomizing 280 patients with evidence of recurrent oligometastatic disease (≤4 lesions) on PSMA-PET/CT 1:1 to either MDT (radiotherapy) alone or MDT + 6 months of ADT. The primary endpoint of this trial is 30 month metastases progression free survival, with key secondary endpoints including overall survival, adverse events, and quality of life outcomes:7

NRG GU011 (PROMETHEAN) is a randomized phase II trial of SBRT with or without relugolix for early PET-detected recurrent oligometastatic prostate cancer. Eligible patients are those with biochemical recurrence following prior curative intent radiation or surgery for localized prostate cancer, PSA < 10 ng/mL, negative conventional imaging, and 1–5 PET-visible metastases (≥1 extra-pelvic). The primary endpoint is conventional imaging-based radiographic progression free survival. Key secondary endpoints include PET-based radiographic progression free survival, overall survival, and quality-of-life outcome measures:

The VA STARPORT study is a phase II/III randomized trial that evaluates the value of adding PET-directed local therapy to standard systemic therapy for biochemically recurrent patients with evidence of ≤5 metastatic lesions on PSMA-PET/CT. PET-directed local therapy is defined as surgery or radiation to all visible metastases and any prostate/prostatectomy bed local recurrences. Systemic therapy will be administered indefinitely, consistent with current guidelines. The primary study endpoint is castrate-resistant prostate cancer-free survival. Key secondary endpoints include clinical and radiographic progression free survival, overall survival, toxicity, and quality of life outcomes:
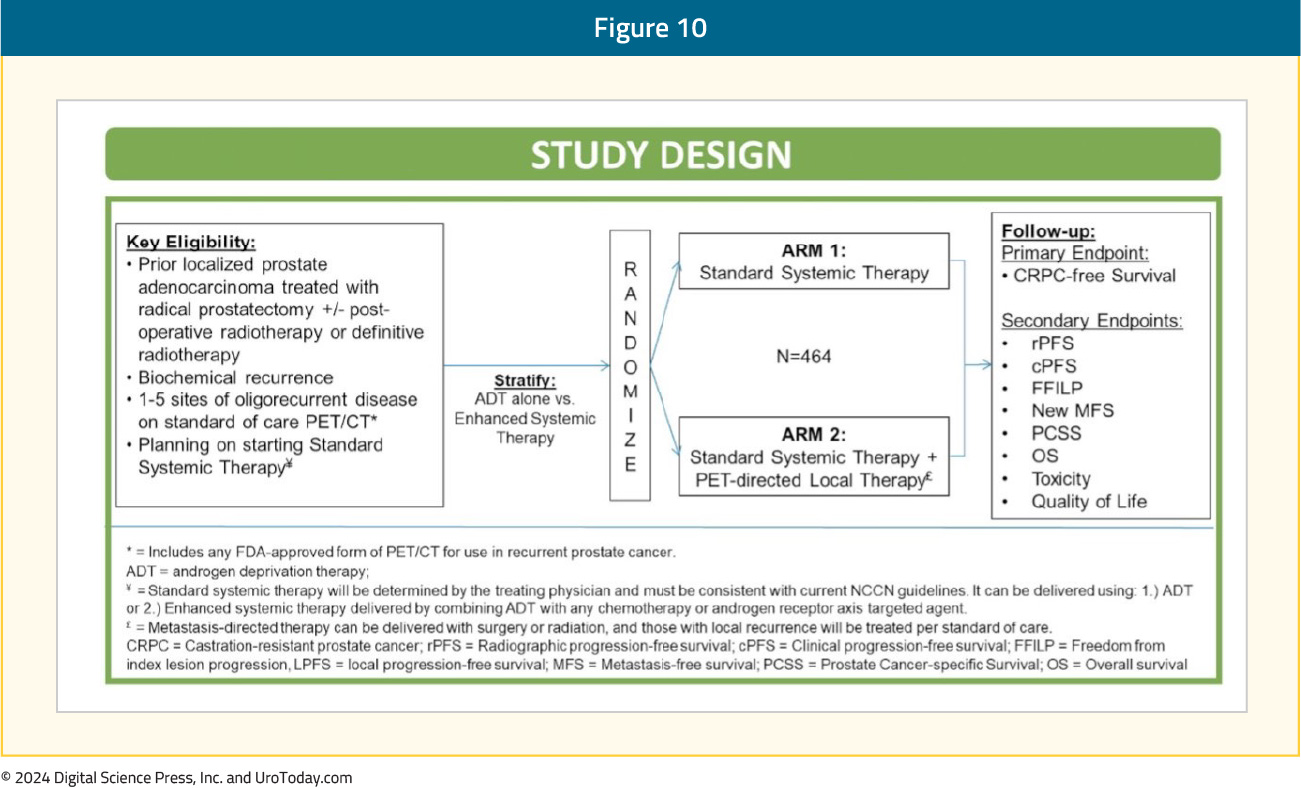
PERSIAN is a randomized phase II trial of recurrent oligometastatic, hormone-sensitive patients (<5 non-visceral lesions). Eligible patients will undergo 1:1 randomization to apalutamide + ADT +/- SBRT to all visible lesions. The primary outcome is 6-month complete biochemical response. The trial design and key secondary endpoints are illustrated below:

The POSTCARD GETUG P13 is a French randomized phase II trial that is randomizing patients with recurrent oligometastatic disease following primary local therapy with curative intent to either SBRT alone (to all visible metastases) or SBRT + durvalumab. Oligometastasis is defined as ≤5 bone or lymph node metastases on 68Ga-PSMA PET/CT or ≤3 bone or lymph node metastases on 18F-choline PET/CT. The primary endpoint is two-year progression-free survival, with key secondary endpoints of androgen deprivation therapy free survival, quality of life, toxicity, prostate cancer specific survival, overall survival, and immune response.8
Incorporating PSMA-PET/CT into the MDT Paradigm
The next ‘frontier’ of MDT trials is incorporating PSMA-PET/CT for staging oligometastatic patients and determining eligibility for MDT. Given the increased sensitivity of PSMA-PET/CT compared to conventional imaging,10 it is likely that this may lead to a ‘stage migration’ phenomenon, where future cohorts of oligometastatic prostate cancer patients, matched for the same number of metastatic lesions, have more favorable prognoses. However, there has been a shift away from defining the ‘upper limit’ of oligometastatic disease by the number of metastatic lesions. A recent survey of participants from the ESTRO-ASTRO consensus conference demonstrated that the majority of respondents concur that the ‘upper limit’ of oligometastatic disease should be defined by the ability to safely deliver curative intent metastasis-directed radiotherapy and not by the number of metastatic lesions.
Existing evidence suggests that PSMA-PET/CT provides additional information in patients with evidence of recurrent oligometastatic disease on conventional imaging. In the ORIOLE trial, as highlighted above, pre-treatment 18F-DCFPyL-PET/CT was performed in all patients assigned to the MDT arm (n = 36). Of the 36 patients treated with SBRT, 16 (44.4%) had baseline PET-avid lesions that were not included in the treatment fields. These patients had significantly worse 6-month progression rates of 38% compared to those without untreated lesions (5%; p = 0.03). Furthermore, those with untreated sites of disease had higher rates of new metastases (per conventional imaging) at 6 months (62.5% versus 15.8%, p = 0.006), and worse median distant metastasis-free survival of 6 versus 29 months (HR: 0.19; 95% CI: 0.07–0.54, p < 0.001). These results highlight the importance of targeting all sites of disease, and the value of PSMA PET/CT for defining this with increased sensitivity compared to conventional imaging.3
A single arm phase II study evaluated the role of MDT (SBRT or surgery) in 37 patients with a rising PSA (0.4–3 ng/ml) following maximal local therapy (radical prostatectomy + adjuvant/salvage radiotherapy) who had not received prior salvage hormonal therapy and had negative conventional imaging, but evidence of oligometastasis on 18F-DCFPyL PET-MR/CT. Ten and 27 patients underwent surgery and SBRT, respectively. At a median follow-up of 16 months, the overall response rate was 60%, including 22% who had biochemical ‘no evidence of disease. One (2.7%) grade 3 toxicity (intra-operative ureteric injury) was observed.11
MDT for De Novo Oligometastatic Hormone-sensitive Prostate Cancer
There are numerous ongoing trials evaluating the role of MDT in combination with systemic therapy in the de novo oligometastatic hormone-sensitive setting. In this section, we will highlight key trials in this space.
SOLAR (NCT03298087) is a single arm phase II trial of 28 patients with de novo M1a/b disease and 1–5 radiographically visible M1 lesions (majority detected via PSMA PET-CT) who all underwent radical local treatment, intensified systemic therapy for six months (leuprolide, abiraterone acetate with prednisone, apalutamide), and metastasis-directed SBRT. Radical local therapy was either radical prostatectomy (n = 12) with lymph node dissection and postoperative radiotherapy (for ≥pT3a, N1, or positive margins) or radical radiotherapy (n=12) directed to the prostate, seminal vesicles, and pelvic lymph nodes. The initial results of this trial were presented at GU ASCO 2024. At a median follow-up of 30 months, 62% of patients completed all planned systemic therapy without dose modification. Of the 22 patients with >6 months follow-up after testosterone recovery, 86% remained free of any progression, defined as an undetectable serum PSA after radical prostatectomy or <2 ng/ml after radical radiotherapy. Grade 2 and 3 toxicities for primary tumor therapy were 46% and 4%, respectively. There were no grade 2–3 toxicities relating to SBRT.12
PLATON (NCT03784755) is a two-arm phase II RCT exploring sequential treatment with ADT +/- chemotherapy followed by cytoreductive prostatectomy or external beam radiotherapy and then SBRT for oligometastases in 410 men. The comparator arm consists of systemic therapy alone, although low-volume men will be allowed to receive conventional local prostate radiotherapy. The primary study outcome is failure-free survival:

IP2-ATLANTA (NCT03763253) is a three-arm phase II randomized trial exploring sequential systemic, local, physical cytoreductive therapy and finally SBRT versus standard of care in 918 men with any-volume metastatic disease. All patients will receive doublet systemic therapy (ADT + docetaxel or enzalutamide or abiraterone). Patients in experimental arm 1 will receive minimally invasive ablative therapy +/- lymph node dissection, whereas those randomized to experimental arm 2 will receive local external beam radiotherapy +/- lymph nodes or radical prostatectomy +/- pelvic lymph node dissection. Patients in both experimental arms will further receive SBRT to oligometastases following receipt of of systemic and local treatment. Patients in the control arm will receive planned systemic therapy only, although patients with low-volume metastases will be eligible for prostate external beam radiotherapy:13

Finally, TERPs (NCT05223803) is a phase II trial of patients with de novo oligometastatic disease (<3 on conventional imaging or <5 on PET/CT) who will be randomized 1:1 to best systemic therapy + primary prostate radiotherapy +/- MDT (SBRT). The primary study endpoint is two-year failure-free survival.14
Conclusions and Future Directions
MDT has emerged as a guideline-recommended treatment option for prostate cancer patients with oligometastatic disease. The evidence to date suggests that MDT can be used in lieu of systemic therapy to delay time-to-hormone therapy or in combination with systemic therapy as a consolidative measure. The latest NCCN guidelines recommend considering SBRT for MDT in oligometastatic patients when ablation is the goal or in oligometastatic patients with limited progression or limited residual disease on otherwise effective systemic therapy (i.e., consolidation), where progression free survival is the goal.
The next ‘frontier’ of clinical trials for MDT will be evaluating its efficacy and safety in patients with PSMA-PET/CT-defined oligometastases and defining its role in the de novo metastatic hormone-sensitive setting. The results of these exciting trials will become available over the next few years.
Published August 2024


