Introduction
The last decade has seen a seismic shift in the treatment landscape of metastatic hormone sensitive prostate cancer (mHSPC). This includes several guideline and FDA approved doublet therapy options, triplet therapy options, and treatment with radiotherapy to the primary tumor:- ADT + apalutamide1,2
- ADT + enzalutamide3-6
- ADT + abiraterone7-9
- ADT + docetaxel + darolutamide10
- Radiotherapy to the primary for low volume disease11,12
Novel Targets in mHSPC
The key to novel targets is identification, and assessment of early phase trial safety and clinical benefit, followed by assessment in phase 2 and 3 randomized clinical trials. Indeed, some of the novel targets in mHSPC are not novel in the traditional sense of the definition, but rather already evaluated/approved in the mCRPC disease space (ie. PARP inhibition, 177Lu-PSMA-617) and looking to “move up” in the prostate cancer continuum into the mHSPC disease space.To date, there is a paucity of genetic, genomic, and transcriptomic data characterizing mHSPC, however progression from localized prostate cancer to mCRPC is secondary to enrichment in alteration of tumor suppressor genes (ie. PTEN, TP53, RB1) genes affecting DNA damage repair (ie. BRCA2, BRCA1, ATM, FANCA), PI3K signaling (ie. PIK3CA, AKT1), chromatic remodeling (ie. KMT2C, KMT2D), and the androgen receptor13-15. mHSPC appears to be more genetically similar to localized prostate cancer rather than mCRPC; previous work suggests that high volume mHSPC has evidence of more genomic instability than low volume mHSPC, particularly with regards to global copy number burden, and frequency of NOTCH pathway, cell cycle, and Wnt signaling alterations.
Figure 1: Therapeutic targets of systemic therapies in advanced prostate cancer16

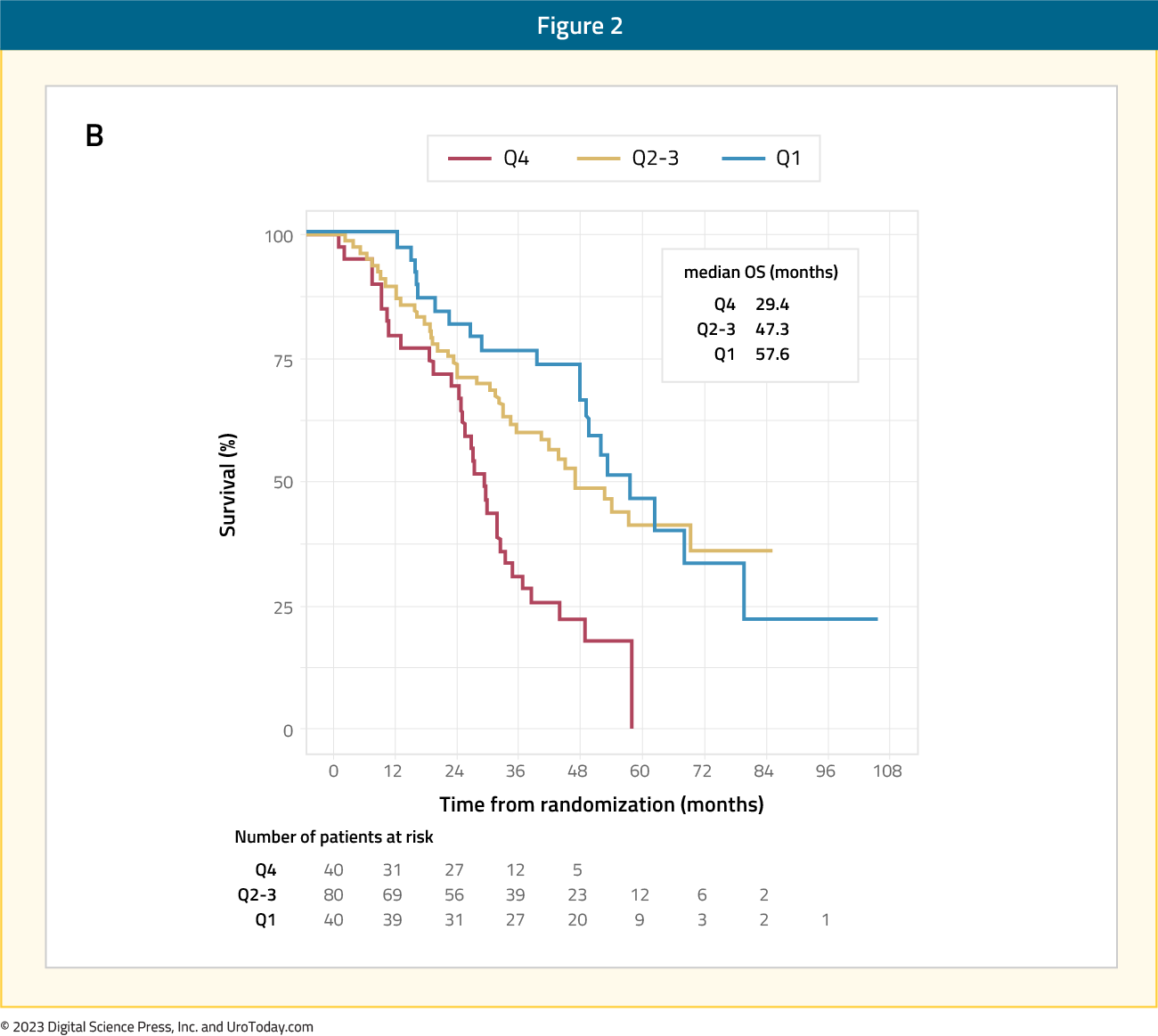
Genomic alterations hold prognostic and predictive value for mHSPC. For example, time to castration resistance is short with alterations in AR, TP53, PTEN, RBI, cell cycle, and MYC pathways17,18. SPOP mutations are associated with death in patients treated with ARSIs, but not docetaxel, for mHSPC. Furthermore, RNA profiling of mHSPC suggests strong biomarker potential. In the CHAARTED study using the Decipher genomic classifier on 160 patients, there were significant differences in genetic signatures compared to localized prostate cancer. Specifically, there was a predominance of luminal B (50%0 and basal subtypes (48%), lower AR activity, and higher Decipher genomic classifier risk19. Luminal B subtype was associated with worse prognosis on ADT but derived significant benefit from the addition of docetaxel (median OS 29.8 vs 52.1 months; HR 0.45, 95% CI 0.25-0.81). Decipher genomic classifier significantly stratified OS, with quartile 1, quartile 2-3, and quartile 4 cut-off subgroups showing 3-year OS rates of 77%, 60%, and 31%, respectively.
Figure 2: Overall survival in CHAARTED stratified by Decipher genomic classifier quartiles
Similar data, presented at ASCO 2020, is available from TITAN with regards to transcriptional subtyping and their association with shorter rPFS, however, there was benefit to adding apalutamide across molecular subtypes. Furthermore, presented at ESMO 2022, the Decipher genomic classifier provided a prognostic role for patients in STAMPEDE treated with or without abiraterone, paralleling the benefit of subgroups of ARSIs. Taken together, transcriptomic subtyping provides prognostic information independent of clinical factors.
The following discussion will highlight several of the exciting ongoing novel target trials in the phase 2 and 3 setting (Table 1). Because of the frequency of BRCA1/2 and HRR mutations in advanced prostate cancer, there are a number of PARP inhibitor trials (AMPLITUDE, TALAPRO-3) under investigation in mHSPC20. Additionally, alterations in the PI3K-Akt signaling pathway has led to the study of combining AKT inhibitors and CDK4/6 inhibitors (CYCLONE-3) with ADT in mHSPC. The prevalence of PSMA expression in mHSPC has spurred investigation of 177Lu-PSMA-617, particularly in combination with chemotherapy (UpFrontPSMA) or in combination with ARSIs (PSMAddition).
CYCLONE-3
CYCLONE 3 is a phase 3, randomized, double-blind, placebo-controlled study of abemaciclib in combination with abiraterone plus prednisone in men with high-risk mHSPC. Abemaciclib is an oral selective inhibitor of cyclin-dependent kinase 4 and 6 (CDK4 & 6) dosed on a continuous schedule, approved for the treatment of advanced or metastatic and certain types of high-risk early stage HR+, HER2- breast cancer. Analogous to the estrogen receptor signaling pathway in breast cancer, there is evidence that the androgen receptor axis activates CDK4 & 6 to sustain prostate cancer cell proliferation, and upregulation of cyclin D1 is a potential mechanism of resistance to novel hormonal agent therapy. In preclinical models, abemaciclib induces cell cycle arrest and prostate tumor growth inhibition. Additionally, ongoing studies are investigating the role of abemaciclib as a single agent and in combination with abiraterone in patients with mCRPC (CYCLONE 1: NCT04408924; CYCLONE 2: NCT03706365).CYCLONE 3 is recruiting approximately 900 patients with high-risk mHSPC defined by ≥4 bone metastasis and/or visceral disease will be randomized in a 1:1 ratio to the abiraterone + prednisone + abemaciclib or placebo arm. Patients will be stratified based on:
- De novo metastatic prostate
- Visceral metastasis
- Prior docetaxel use for mHSPC
Figure 3: The trial design for CYCLONE 3

The primary endpoint is investigator-assessed rPFS, and key secondary endpoints include: rPFS assessed by blinded independent central review, CRPC-free survival, overall survival, time to pain progression, time to deterioration in health-related quality of life, safety, and pharmacokinetics. This study is actively enrolling in 270 sites across 25 countries.
UpFrontPSMA21
This trial is an open-label, randomized, multicenter, phase II trial recruiting 140 patients at 12 Australian centers. There are several important eligibility criteria:- Prostate cancer with a histological diagnosis within 12 weeks of screening
- PSA >10ng/mL at diagnosis
- ≤ 4 weeks on ADT
- Metastatic disease on CT and/or bone scan
- High-volume PSMA-avid disease with the SUVmax > 15
- Absence of extensive discordant FDG-positive, PSMA-negative disease
Figure 4: Trial design for UpFrontPSMA

PSMAddition
PSMAddition is a phase 3 trial comparing 177Lu-PSMA-617 + standard of care and standard of care alone in patients mHSPC. This is an international, prospective, open-label, randomized, phase 3 trial that will enroll an estimated 1,126 patients with treatment-naïve or minimally treated PSMA-positive mHSPC. Eligible patients must also have PSMA-positive disease (determined by 68Ga-PSMA-11 PET/CT), ECOG performance status of 0 to 2, and adequate major organ function. Patients will be randomized 1:1 to receive 177Lu-PSMA-617 (7.4 GBq every 6 weeks for a maximum of 6 cycles) + standard of care or standard of care alone (an androgen receptor pathway inhibitor and ADT).Figure 5: The trial design for PSMAddition

Stratification factors are tumor volume (high/low), age (≥ 70/ < 70 years), and previous/planned prostatectomy or radiation treatment of the primary prostate tumor (yes/no). The primary endpoint is rPFS, as assessed by blinded independent centralized review. Upon centrally confirmed radiographic progression, participants in the standard of care-only arm can cross over to the 177Lu-PSMA-617 arm.
Secondary endpoints include:
- Overall survival
- Proportion of patients with a PSA decline of ≥ 90% from baseline
- Time to development of metastatic castration-resistant prostate cancer
- Composite PFS (radiographic, clinical, or PSA progression)
- Safety
- Tolerability
- Health-related quality of life
PROMETHEAN
The NRG-GU011/PROMETHEAN study is a phase II double-blinded, placebo-controlled trial of prostate oligometastatic radiotherapy with or without ADT. This trial will assess the knowledge gap about timing and benefit vs toxicity of ADT with stereotactic ablative radiotherapy (SABR) in early oligometastatic prostate cancer. PROMETHEAN will randomize patients to SABR + 6 months relugolix versus SABR + 6 months placebo. Relugolix allows for rapid testosterone recovery and is associated with fewer cardiovascular events when compared to leuprolide and is hypothesized to have low late ADT toxicity.Figure 6: The trial design for NRG-GU011/PROMETHEAN:
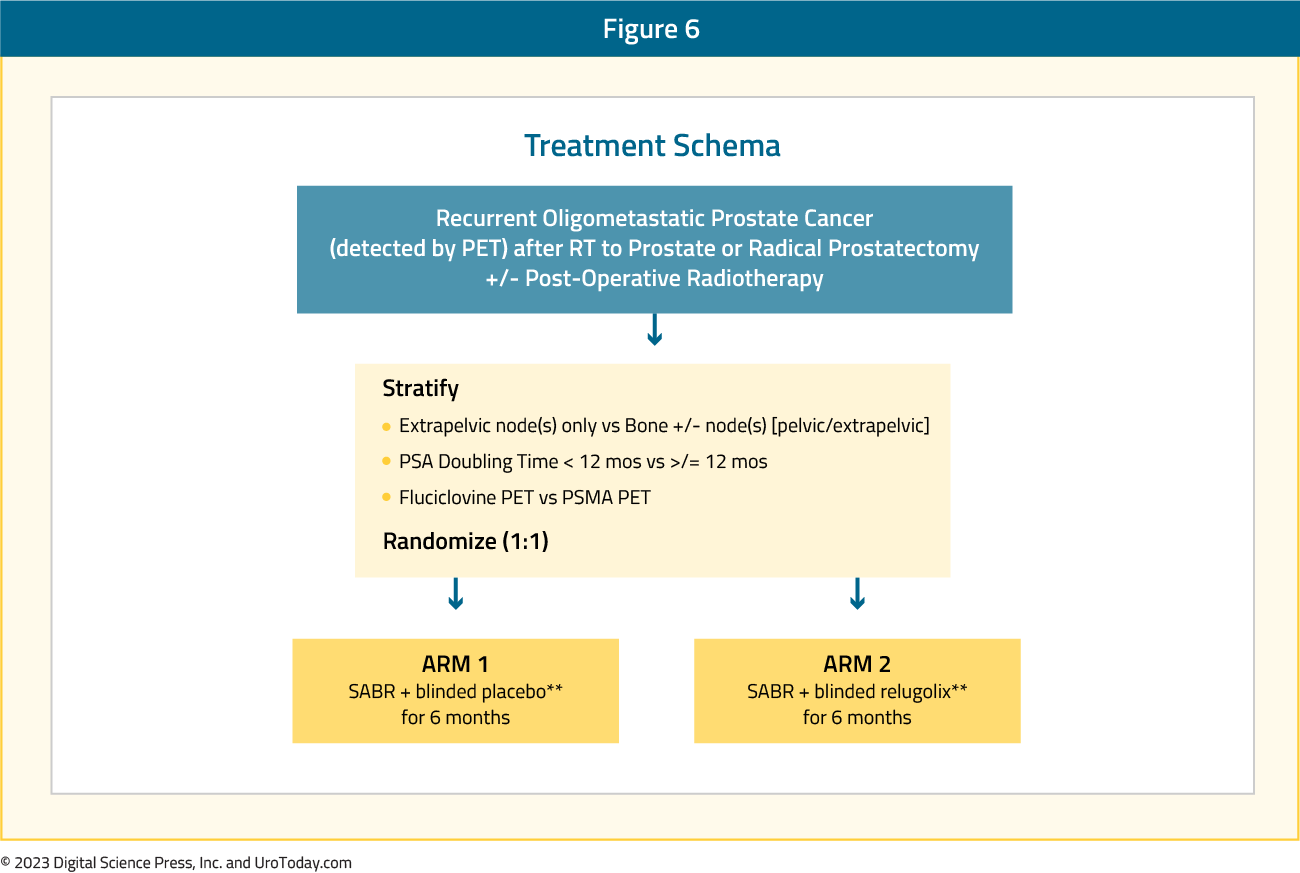
Eligible patients have biochemical recurrence after prior curative intent radiation or surgery for localized prostate cancer, PSA < 10 ng/mL, negative conventional imaging, and 1-5 PET-evident metastases (≥1 extrapelvic). SABR is delivered in 1-5 fractions to BED >100 Gy1.5, followed by placebo or relugolix 120 mg daily for 6 months. The primary endpoint is conventional imaging radiographic progression-free survival, with several key secondary endpoints:
- PET-based radiographic progression-free survival
- Sexual and hormonal quality of life assessed by EPIC-26
- Other QoL measures from EQ5D-5L, EORTC QLQ-3,0 and PROMIS Fatigue
- Between-group comparisons of time to salvage therapy, castration-resistance, local progression (SABR-targeted lesion), biochemical progression, distant metastases, prostate cancer-specific mortality, metastasis-free survival, and overall survival

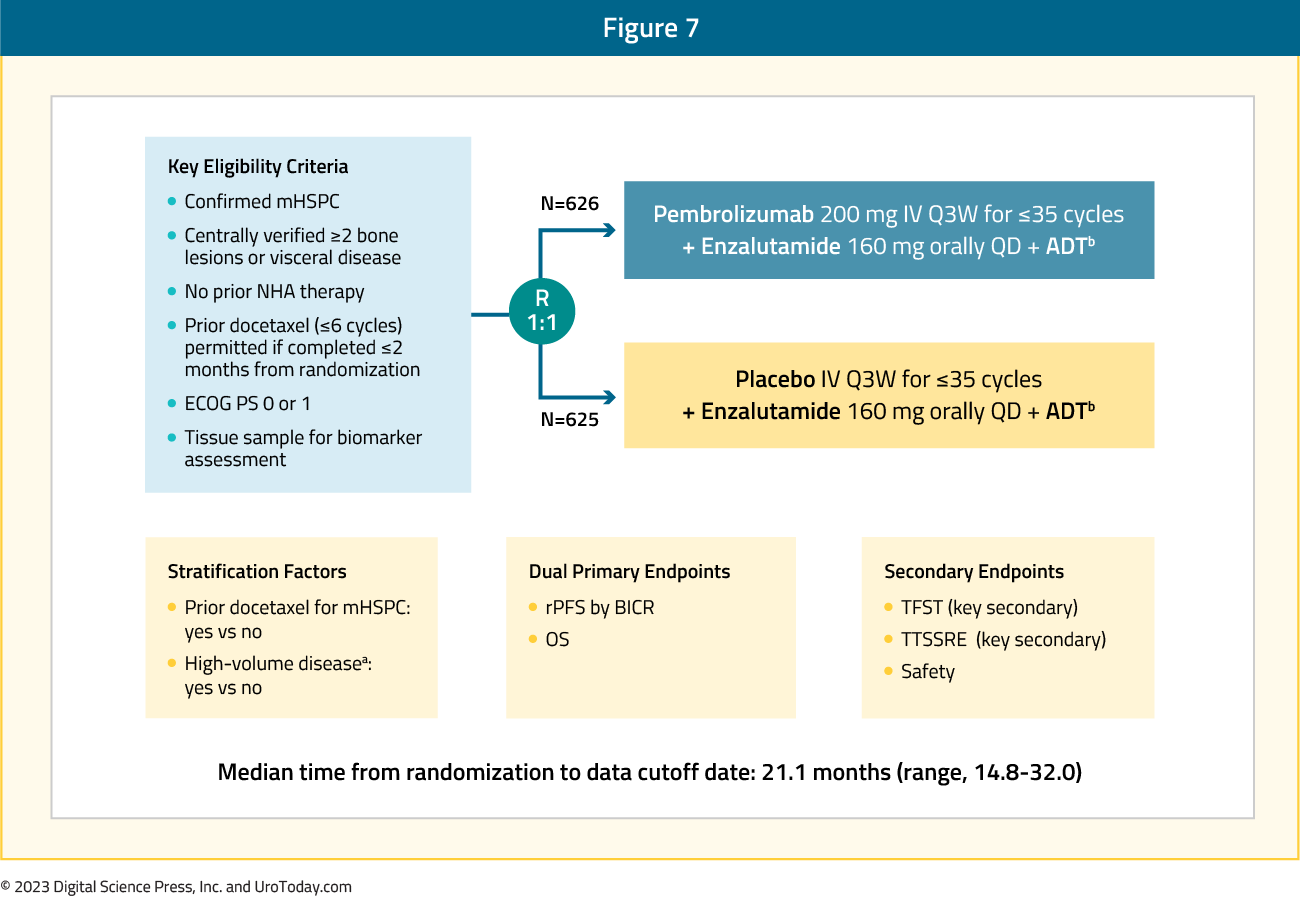
The results of the KEYNOTE-991 were presented at ESMO 2023, investigating pembrolizumab or placebo + enzalutamide + ADT in patients with next-generation hormonal agent-naïve mHSPC. For this trial, eligible patients had confirmed mHSPC (≥2 bone lesions and/or visceral disease, verified centrally), no prior next-generation hormonal agent, and had completed any prior docetaxel (≤6 cycles) ≤2 months from randomization. Patients were randomized 1:1 to receive pembrolizumab 200 mg or placebo IV every 3 weeks for ≤35 cycles + enzalutamide 160 mg orally daily + continuous ADT (if no history of bilateral orchiectomy).
Figure 7: The trial design for KEYNOTE-991:
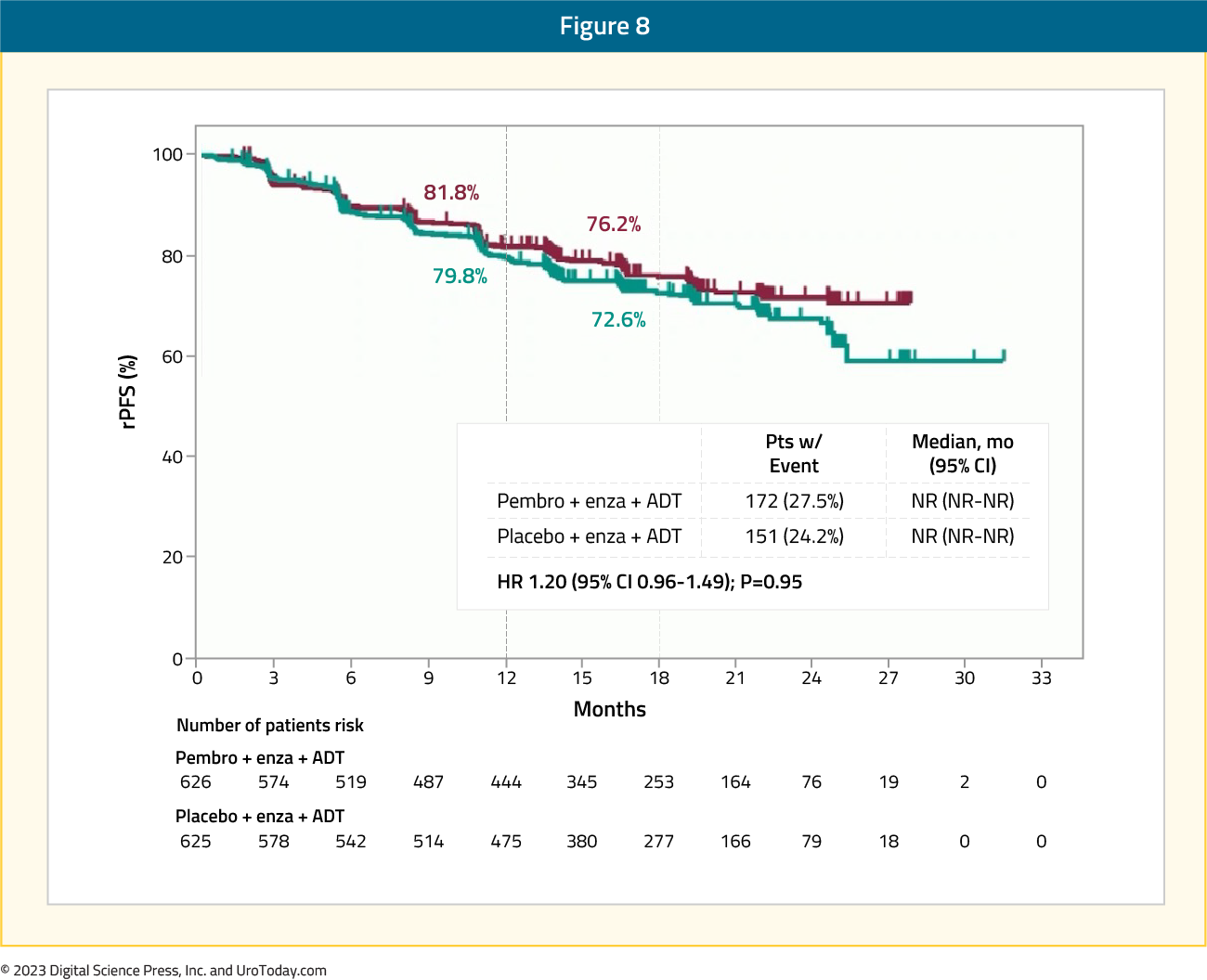
Dual primary endpoints were rPFS per PCWG-modified RECIST 1.1 by blinded independent central review and overall survival (OS). There were 626 patients randomized to pembrolizumab + enzalutamide and 625 to placebo + enzalutamide. Over a median follow-up time at first prespecified interim analysis of 21.1 months (range: 14.8−32.0), the primary endpoint of rPFS for pembrolizumab + enzalutamide vs placebo + enzalutamide was not met (median NR vs NR, HR 1.20, 95% CI 0.96−1.49):
Figure 8: rPFS in the KEYNOTE-991 trial
The median OS was NR vs NR (HR 1.16, 95% CI 0.88−1.53) and not statistically significant. Based on the interim results, KEYNOTE-991 was stopped for futility. As such, the role of immune checkpoint inhibition in mHSPC (and advanced prostate cancer in general) remains unclear, and further studies are needed to identify patients most likely to benefit.
Treatment Development/Prognostication
With a plethora of options available for first line treatment of mHSPC, how we delineate, develop a treatment plan, and prognosticate who derives treatment benefit is of the utmost importance. Beyond the usual clinical parameters we use for treatment selection, recent advances in artificial intelligence and machine learning have led to exciting advances in understanding which patients may benefit from treatment, treatment intensification vs de-intensification, etc.The ArteraAI Prostate Test is an artificial intelligence-derived digital pathology based biomarker to predict the benefit of long-term ADT addition to radiotherapy in men with localized, high-risk prostate cancer. At ASCO 2023, results of a pooled analysis of multiple phase III NRG/RTOG trials demonstrated that the ArteraAI Prostate Test was a significant predictor of the utility of long-term ADT use in this cohort (interaction p=0.04). Patients in the biomarker negative group (n = 407) derived no benefit from long-term ADT use, compared to short-term ADT use (HR 1.06, 95% CI 0.61 – 1.84). Conversely, patients in the biomarker positive group had significant improvements in the distant metastasis rates with long-term ADT (HR 0.55, 95% CI 0.41 – 0.73).
Figure 9: Distant metastasis risk in the biomarker negative versus positive groups evaluated by the ArteraAI Prostate Test
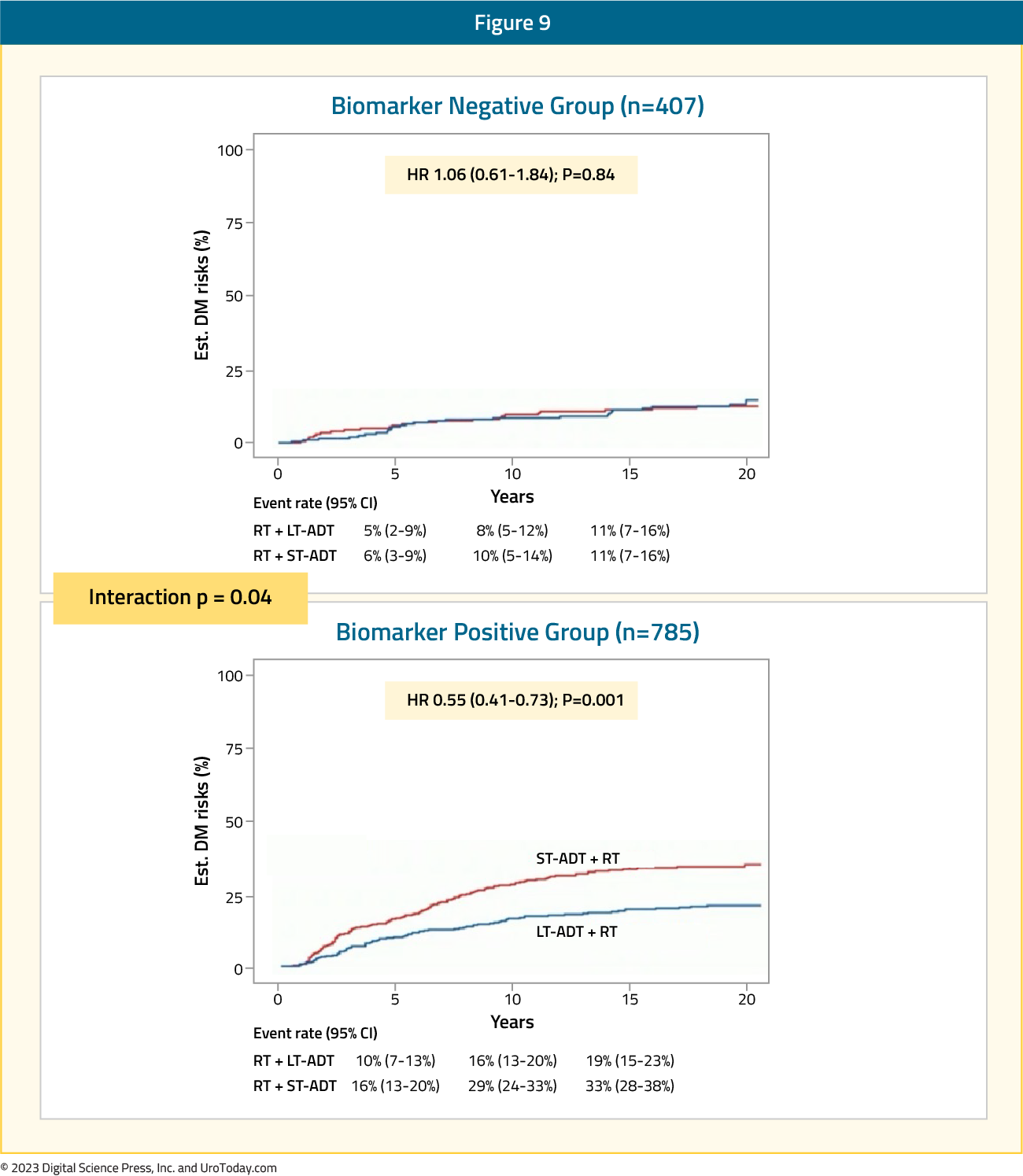
Accordingly, approximately 1/3 of men with high-risk prostate cancer could have safely avoided long-term ADT based on the results of this predictive biomarker analysis:
Specific to the mHSPC disease space, ArteraAI Prostate Test was evaluated in
high-risk M0/M1 prostate cancer starting ADT in the docetaxel or abiraterone phase 3 STAMPEDE trials. Presented at ESMO 2023, the multimodal artificial intelligence model used digitized whole scan images from new H&E core prostate biopsies, local Gleason score, tumor stage, age, and pre-ADT serum PSA
Figure 10: The ArteraAI Prostate Test STAMPEDE study design:
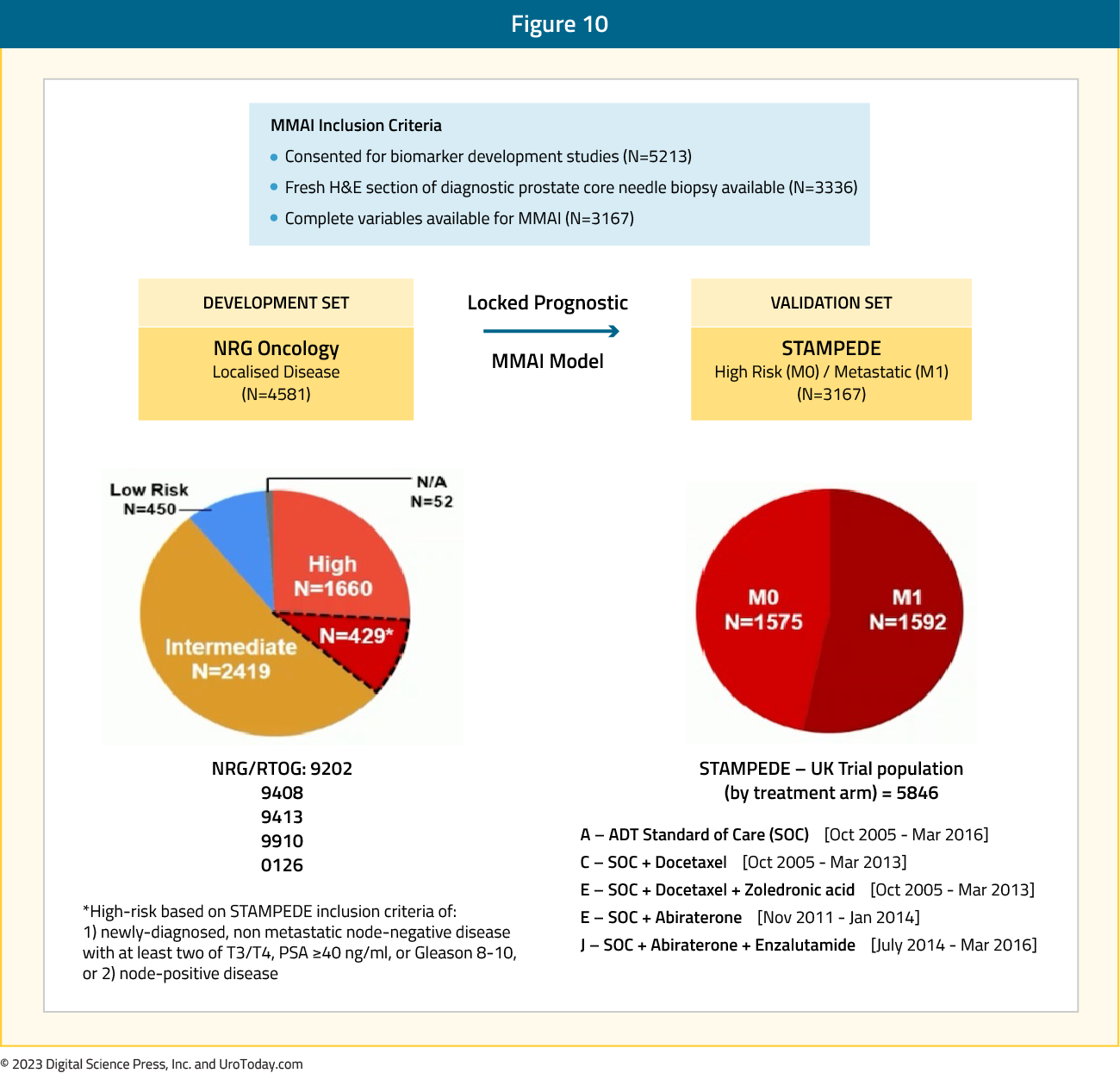
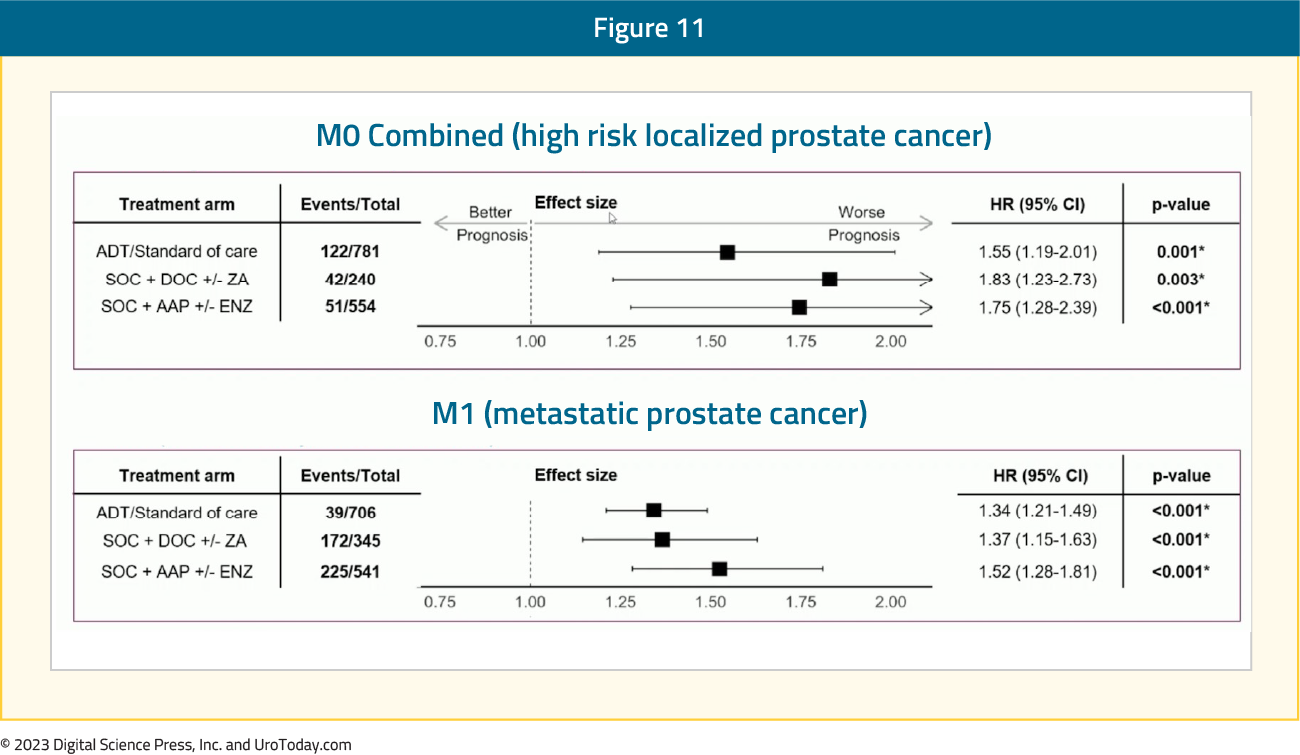
Among patients recruited to ADT +/- radiotherapy alone or + docetaxel +/-zoledronic acid or + abiraterone, 3,336 (1,575 M0, 1,592 M1) had complete data. ArteraAI Prostate Test was strongly associated with:
- PCSM (HR 1.67, 95% CI 1.50-1.84)
- Overall survival (HR 1.51, 95% CI 1.40-1.63)
- Failure-free survival (HR 1.48, 95% CI 1.38-1.59)
- Metastatic progression-free survival (HR 1.59, 95% CI 1.46-1.73)
Additionally, the ArteraAI Prostate Test quartile 4 versus quartile 1-3 had more PCSM events at 5 years: 16% (95% CI 12-19%) versus 5% (95% CI 4-7%) in M0, 58% (95% CI 53-63%) versus 39% (95% CI 36-42%) in M1.
Figure 12: M0 and M1 cohorts stratified by the ArteraAI Prostate Test quartiles on the outcome of PCSM
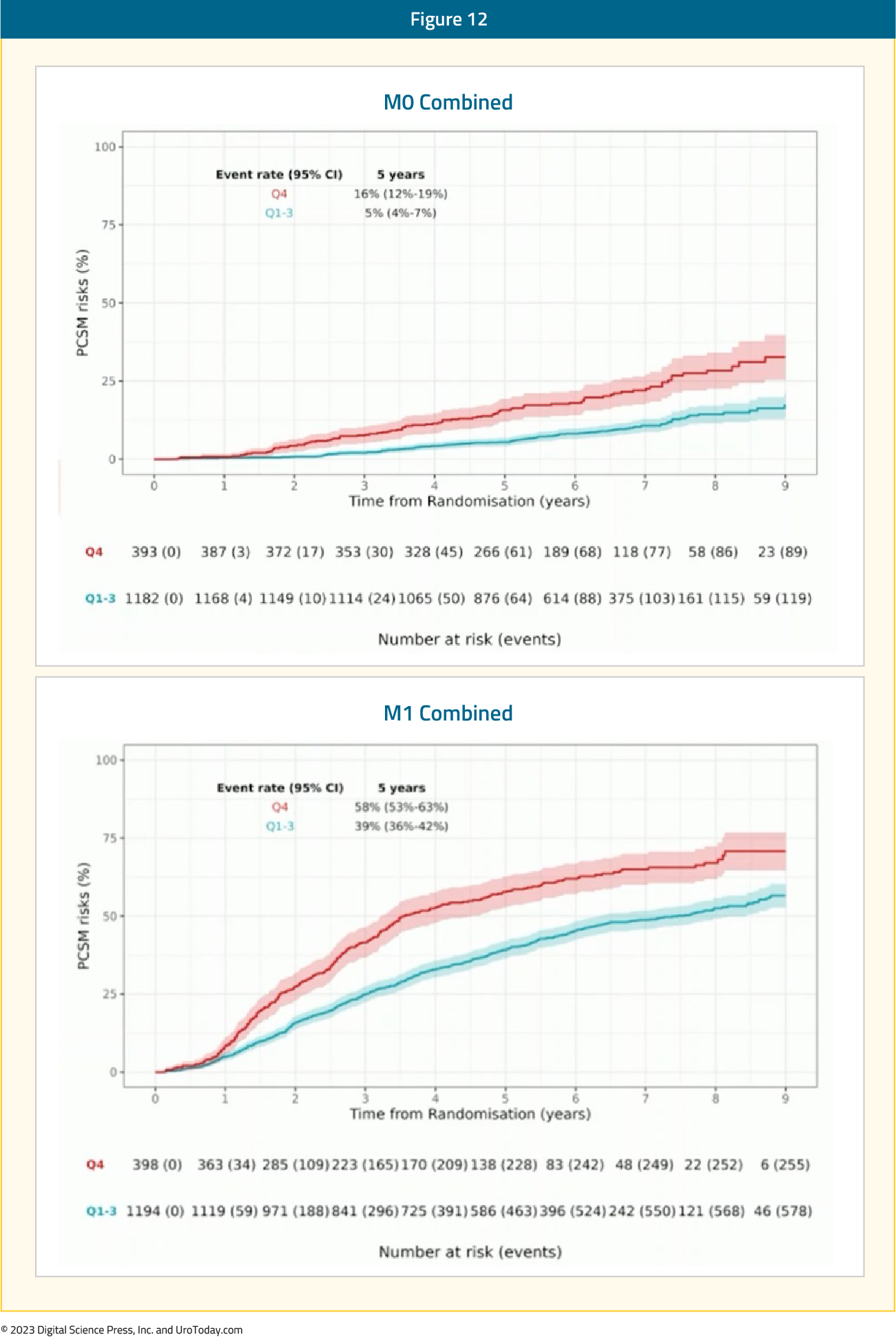
Thus, the ArteraAI Prostate Test identified poor prognostic features in prostate biopsies from high-risk M0 and M1 prostate cancer, which may be used to develop aggressive treatment plans for those at highest risk in the mHSPC disease space.
Conclusions
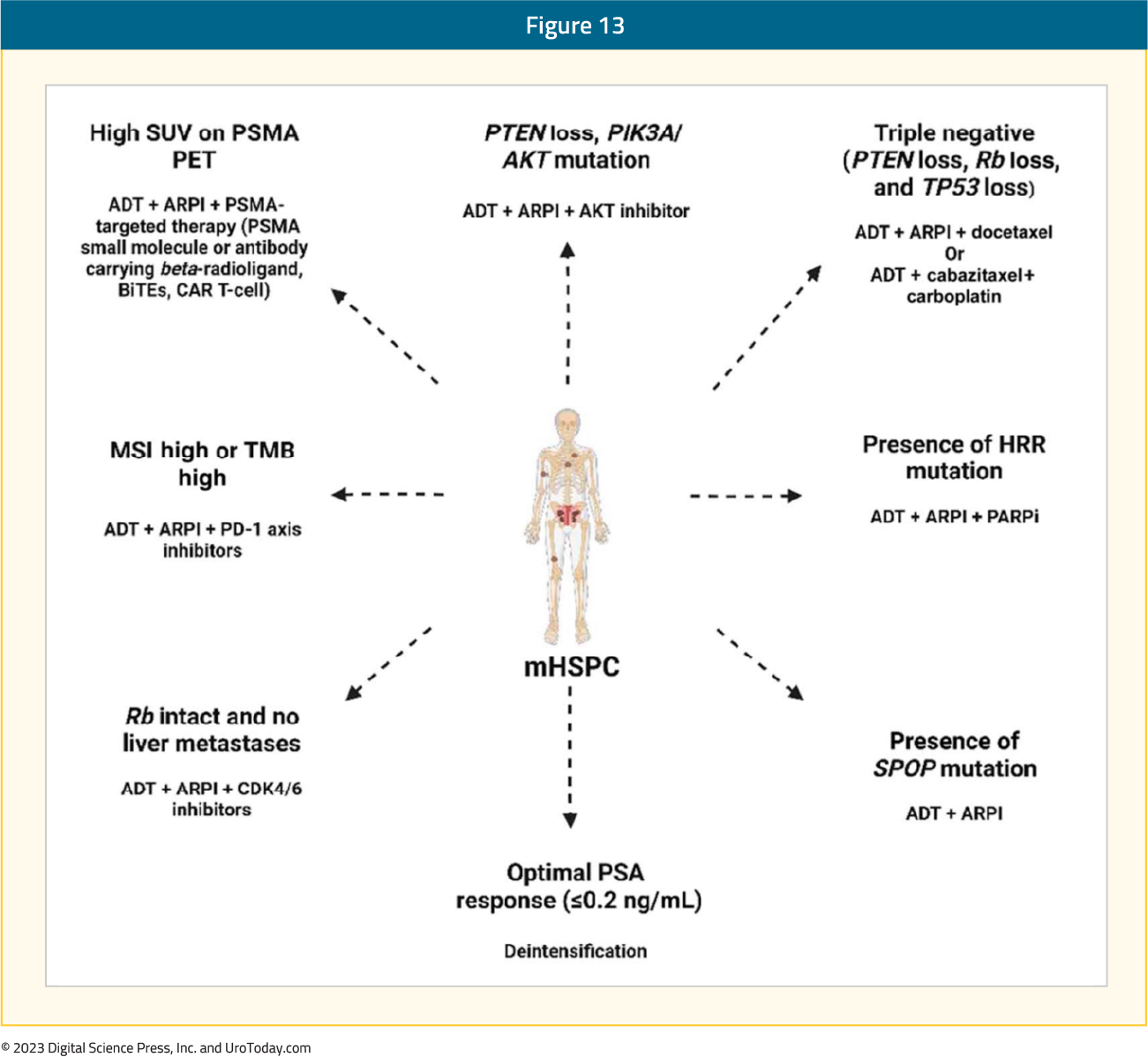
Despite excellent double and triplet therapy options, novel targets and treatment development with enhanced prognostication for mHSPC patients is needed. There are exciting phase II and III trials ongoing that will assess truly novel targets, as well as targets that have had success in patients with mCRPC, and are now being evaluated in patients with mHSPC. Artificial intelligence is a novel modality for prognosticating those with poor outcomes based on digital histopathology slides, with recent outcomes reported in the mHSPC space. The goal, in the hopefully near future, will be to have precision therapy approaches in mHSPC.Figure 13: Potential precision therapy approaches in mHSPC16
Related Content: New Pathways for Treating Metastatic Castration-Resistant Prostate Cancer (mCRPC)
Published November 2023
Part of an Independent Medical Education Initiative Supported by LOXO@Lilly


