Introduction
In November 2023, the United States Food and Drug Administration (FDA) approved enzalutamide, with or without concurrent leuprolide therapy, for non-metastatic hormone-sensitive prostate cancer (M0 HSPC) patients with biochemical recurrence at high risk for metastasis.1 This drug approval followed the publication of the EMBARK trial, a randomized phase III trial of biochemically recurrent patients who had high-risk disease, defined by a PSA doubling time (PSADT) ≤9 months and a PSA level of ≥2 ng/mL above nadir following radiation therapy or ≥1 ng/mL after radical prostatectomy, with or without postoperative radiation therapy. Patients in this trial were randomized 1:1:1 to combination enzalutamide + leuprolide, leuprolide monotherapy, or enzalutamide monotherapy. This trial met its primary endpoint of improved 5-year metastasis-free survival with the combination of enzalutamide + leuprolide (87.3% versus 71.4% for leuprolide alone; HR 0.42, p < 0.001). Similarly, enzalutamide monotherapy was associated with superior 5-year metastasis-free survival rates, compared to leuprolide monotherapy (80% versus 71%; HR 0.63, p = 0.005).2While both enzalutamide monotherapy and combination therapy with leuprolide have demonstrated promising efficacy outcomes for these high-risk patients, it is important to consider the safety profiles of these drugs and their impact on health-related quality of life measures. These patients have a prolonged natural history,3 and thus their survivorship care must remain of utmost importance.
In this Center of Excellence article, we will discuss the health-related quality of life effects of the different treatment arms of the EMBARK trial and highlight important functional outcomes considerations, particularly with regard to sexual function.
EMBARK
In the EMBARK trial, patient-reported outcomes were assessed at baseline and every 12 weeks thereafter until disease progression or completion of data gathering. For each patient-reported outcomes instrument, change from baseline (i.e., post-baseline value minus baseline value) was calculated for each assessment of a domain. Health-related quality of life was measured using Brief Pain Inventory–Short Form (BPI-SF), Functional Assessment of Cancer Therapy-Prostate (FACT-P), Quality of Life Questionnaire–Prostate 25 (QLQ-PR25), and European Quality of Life 5-Dimensions 5-Levels health questionnaire (EQ-5D-5L) scores.4At baseline, the questionnaire completion rates were ≥90% and remained high (≥85%) up to week 205. The baseline patient-reported outcome scores indicated low levels of pain and high health-related quality of life scores, suggesting that most patients were asymptomatic at baseline. However, sexual activity scores were low likely secondary to prior treatments received (50% radical prostatectomy and radiotherapy, 25% radical prostatectomy alone, 25% radiotherapy alone). Very few patients reported hormonal therapy-related symptoms at baseline, as only 31% had received prior hormones and none within the preceding nine months.
Overall, the patient-reported outcome scores remained stable throughout the trial for most patients in all groups, suggesting maintenance of the high baseline health-related quality of life and low baseline symptoms across all groups, with no clinically meaningful differences overall across the groups.
Key Outcomes: Worst Pain in Prior 24 Hours and Functional Status
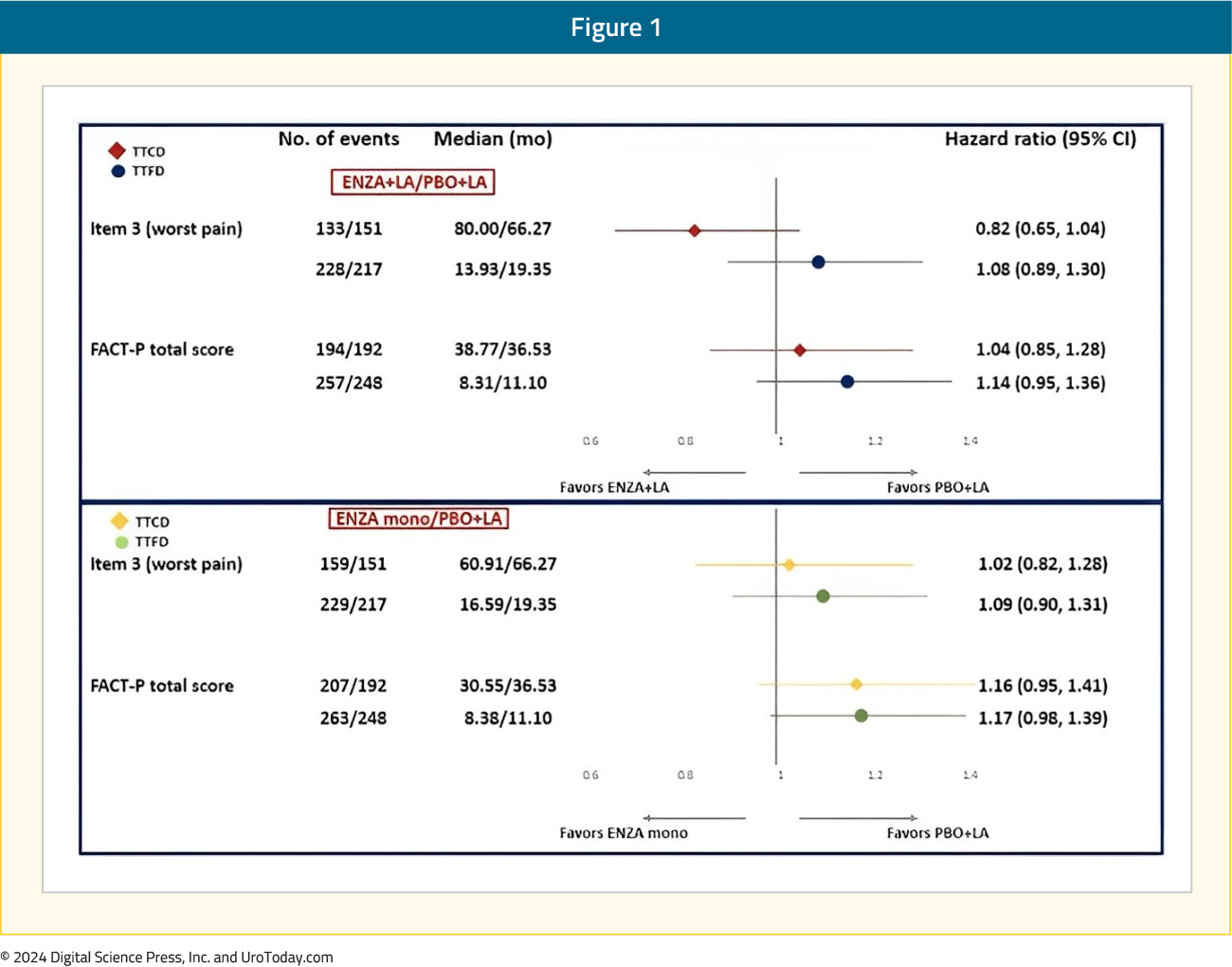
There were no differences in either the time to first or confirmed clinically meaningful deteriorations in worst pain in the past 24 hours (BPI-SF item 3) or in functional status measured using FACT-P total score:
FACT-P Subdomains
The FACT-P total score longitudinally worsened in all three arms, with no clinically meaningful differences between the three arms (leuprolide alone: -3.9; enzalutamide combination: -5.7; enzalutamide monotherapy: -6.4):
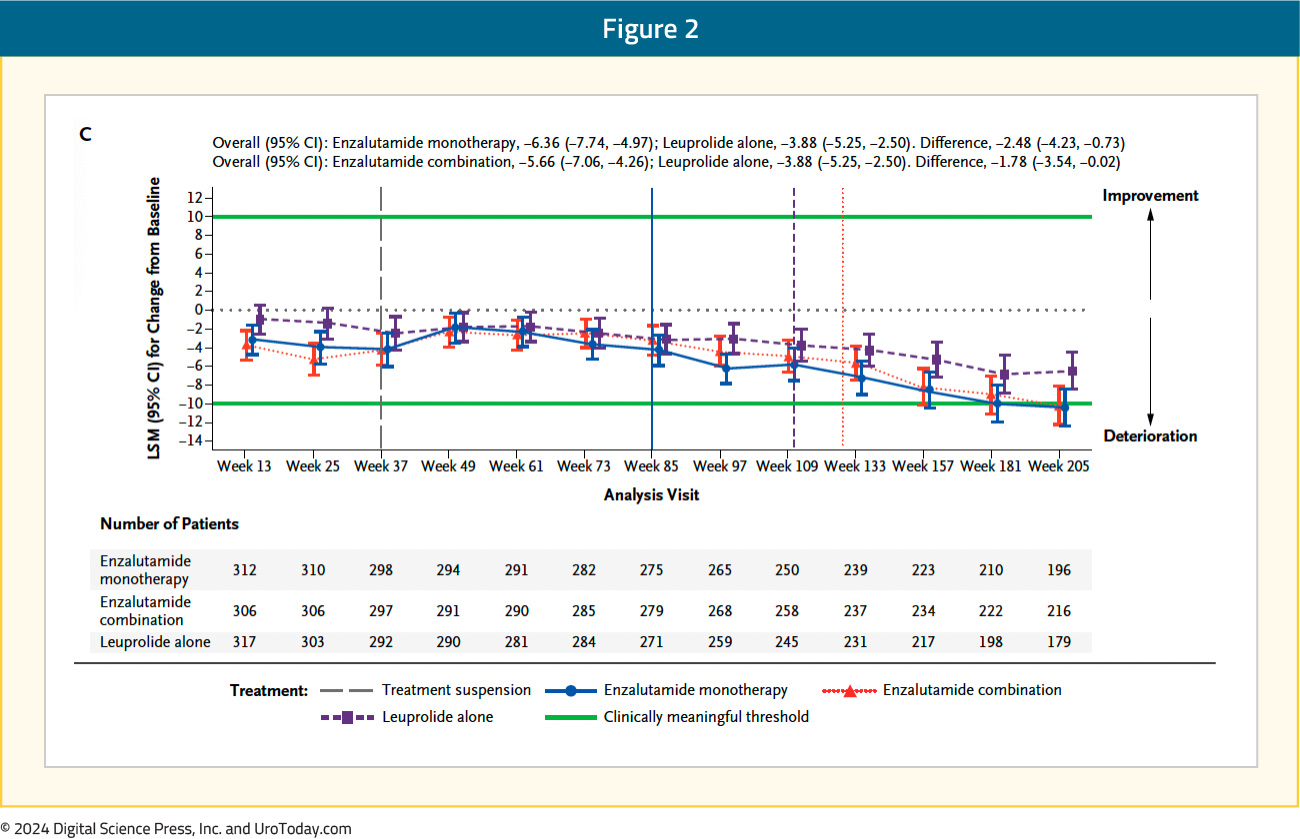
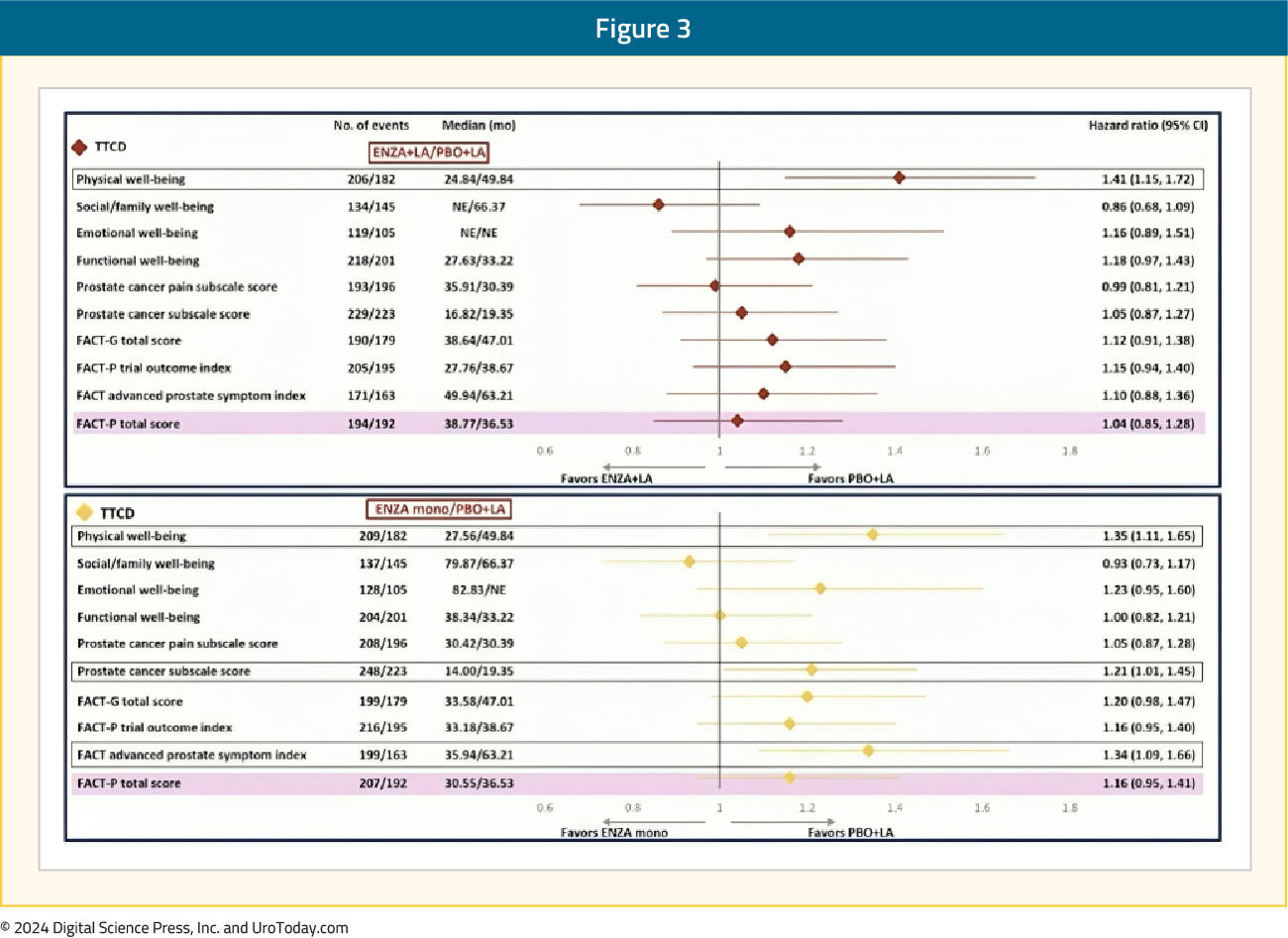
Within the individual FACT-P domains, patients in both the enzalutamide combination and monotherapy arms had significantly shorter (i.e., worse) time to confirm clinically meaningful deterioration in the physical well-being score, compared to leuprolide alone. Time to confirmed clinically meaningful deterioration in physical well-being score was 49.8 months with leuprolide, compared to 24.8 and 27.6 months for enzalutamide combination and monotherapy, respectively (HRs 1.41 and 1.35, respectively).
Patients in the enzalutamide monotherapy arm had a significantly worse median time to first clinically meaningful deterioration in the prostate cancer subscale score (HR 1.21) and advanced prostate symptom index (HR 1.34), compared to leuprolide-only therapy. There were otherwise no significant differences observed in the times to first or confirmed clinically meaningful deteriorations for the other FACT-P domains, including social/family, emotional, and functional well-being and prostate cancer pain domains:
Sexual Function and Hormonal Symptoms
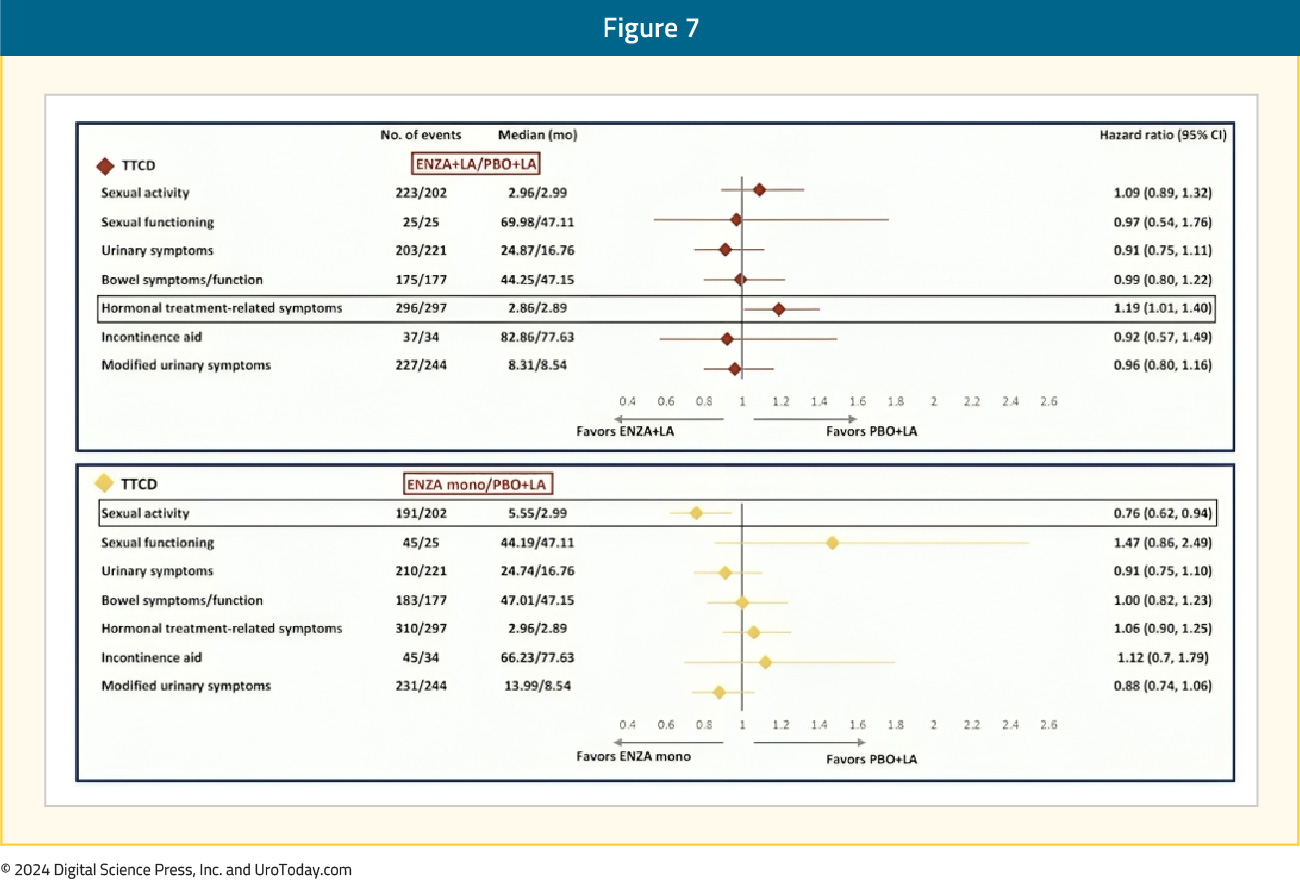
With regards to QLQ-PR25, the time to confirmed clinically meaningful deterioration of composite sexual activity was significantly longer (i.e., better) with enzalutamide monotherapy, compared to leuprolide-only therapy (median: 5.6 versus 3 months; HR 0.76, 95% CI 0.62–0.94). The longitudinal changes in sexual function are summarized below, with enzalutamide monotherapy denoted below in blue:
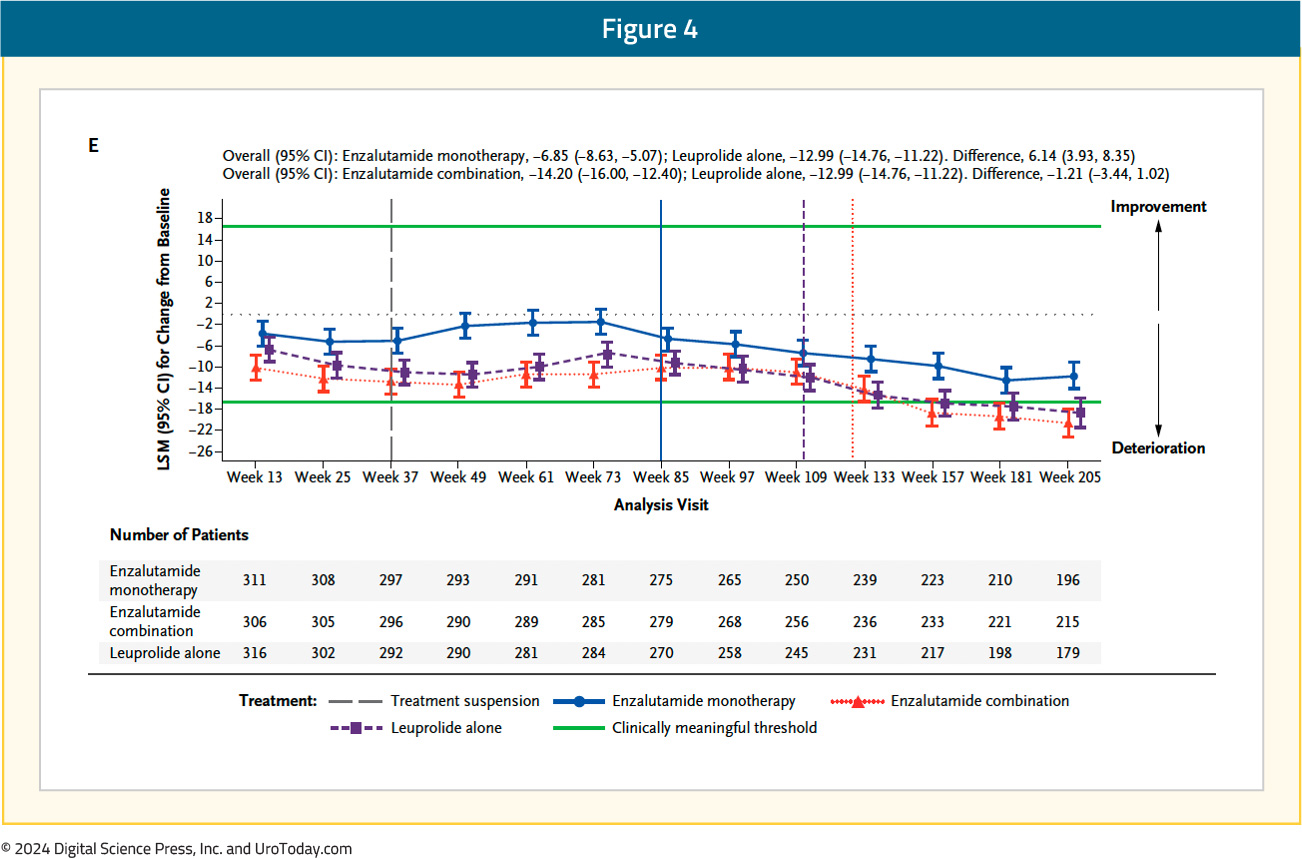
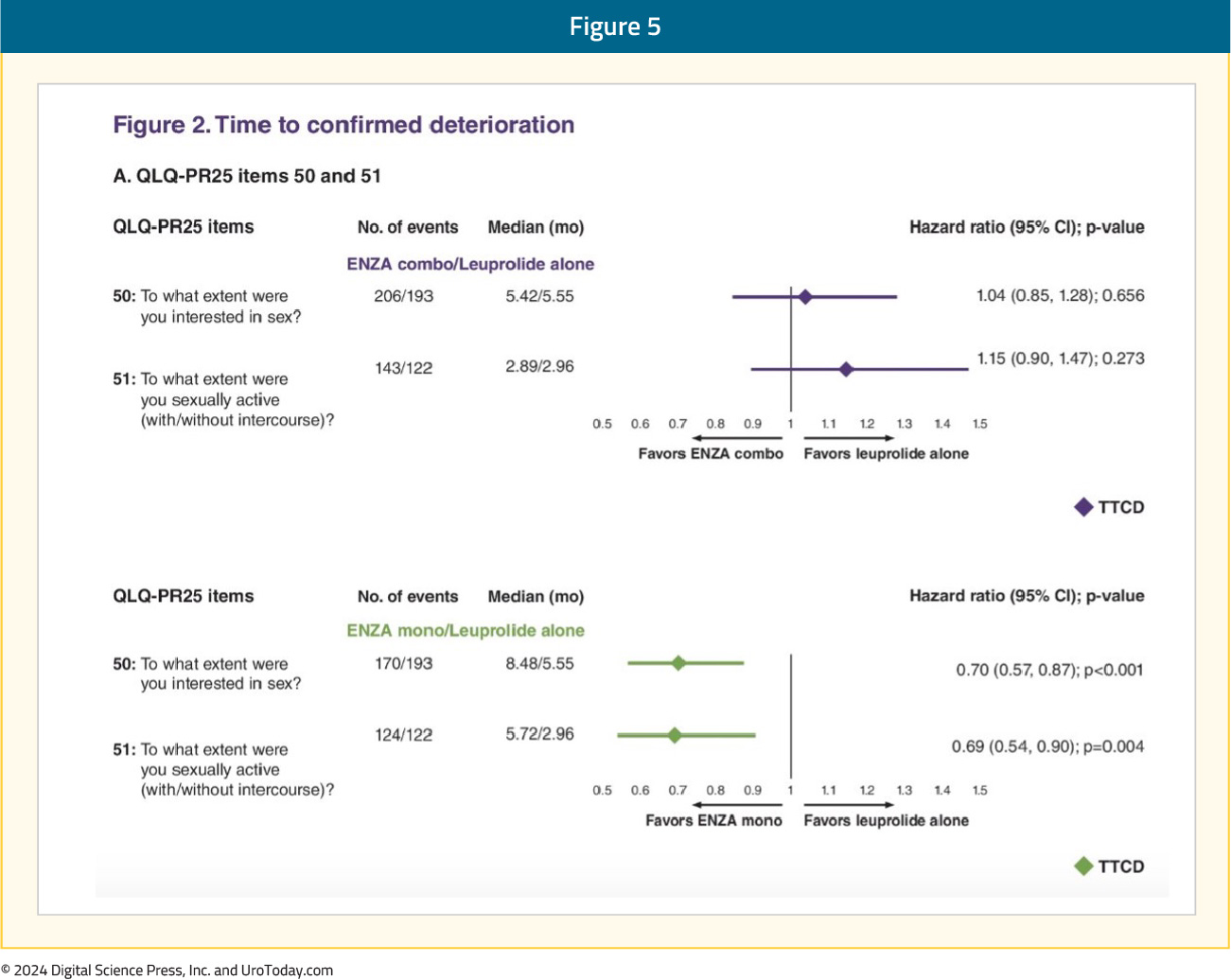
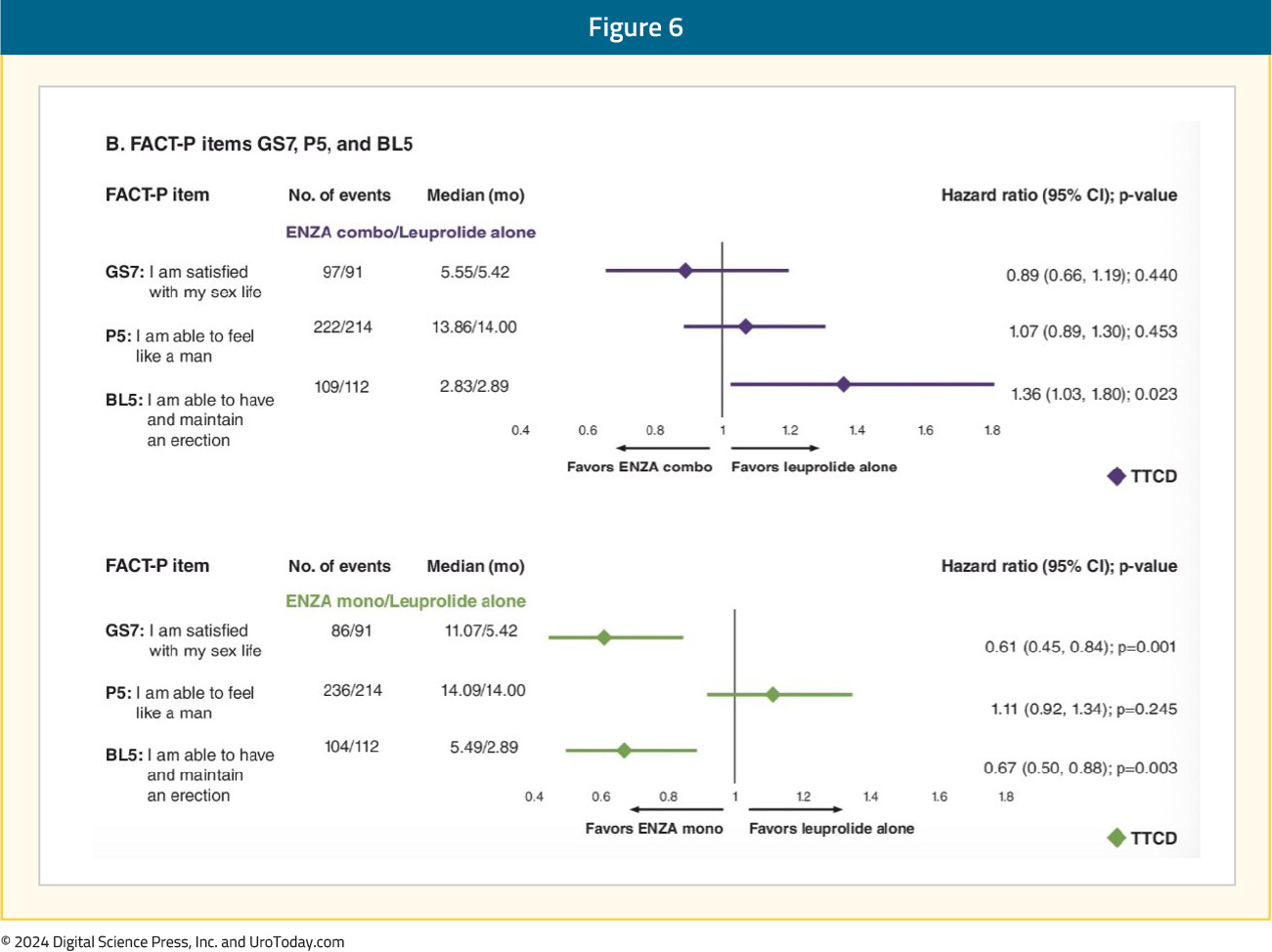
An individual item-level analysis of sexual function was presented at ASCO GU 2024 and demonstrated that enzalutamide monotherapy, compared to leuprolide monotherapy, was associated with significantly longer times to deterioration of sexual interest and sexual activity. Patients in the enzalutamide monotherapy arm had significantly higher satisfaction with their sexual life and ability to achieve and maintain erections. In the comparison of enzalutamide combination to leuprolide monotherapy, patients in the enzalutamide combination arm had significantly worse ability to achieve and maintain erections:5
The time to confirmed clinically meaningful deterioration in hormonal treatment-related symptoms with enzalutamide combination was significantly worse compared to leuprolide alone (HR 1.19, 95% CI 1.01–1.40), although there were minimal differences in the median times to deterioration (2.86 versus 2.89 months):
Safety
No new safety signals were observed with the combination of enzalutamide and leuprolide. Grade ≥3 adverse events were observed in 43-50% of patients across the three arms. The most common adverse events (occurring in ≥10% of patients) in the enzalutamide combination group and the leuprolide-alone group were hot flashes (57–69%) and fatigue (33–43%). The most common adverse events in the monotherapy group were gynecomastia (45% versus 8–9% in the two other arms), hot flashes (22%), and fatigue (47%). Notable adverse events in the enzalutamide monotherapy group were nipple pain (15% versus 1–3% in the other two arms) and breast tenderness (14% versus 1% in the other two arms). Fractures were more common in the enzalutamide combination group (18.4%), compared to the leuprolide-alone group (14%) and the enzalutamide monotherapy group (11%).
Treatment was discontinued due to adverse events in 21% of patients in the enzalutamide combination group, 10% of patients in the leuprolide-alone group, and 18% of patients in the enzalutamide monotherapy arm. The most common adverse event leading to discontinuation in all three arms was fatigue.
In a post-hoc analysis of outcomes stratified by age presented at ESMO 2024, serious adverse events were more common in patients aged ≥70 years vs <70 years for enzalutamide + leuprolide (45.0% vs 25.3%), leuprolide alone (36.2% vs 27.1%), and enzalutamide monotherapy (43.9% vs 30.4%). For clustered treatment emergent adverse events of special interest of any grade, musculoskeletal events, fatigue, and hypertension were common across all age and treatment groups.
Between-arms differences are summarized below (all comparisons are to leuprolide-only therapy):
- Physical well-being score: Significantly worse time to confirmed clinically meaningful deterioration of physical well-being score for both enzalutamide combination and monotherapy arms (leuprolide: 50 months; enzalutamide combination/monotherapy: 25–28 months). These scores likely reflect the higher incidence of fatigue with enzalutamide combination (43%) or monotherapy (47%), versus leuprolide-only therapy (33%).
- Prostate cancer subscale score and advanced prostate symptom index: Worse for enzalutamide monotherapy patients
- Social/family, emotional, and functional well-being and prostate cancer pain FACT-P domains: No differences between the arms
- Sexual function: Deteriorates in all three arms. Significantly longer time to confirmed clinically meaningful deterioration of composite sexual activity with enzalutamide monotherapy (5.6 versus 3 months). Patients treated with enzalutamide monotherapy reported superior sexual interest and activity and had higher satisfaction with their sexual life and ability to achieve and maintain erections. Enzalutamide combination therapy patients had significantly worse ability to achieve and maintain erections.
- Hormonal treatment-related symptoms: Enzalutamide combination patients had a significantly worse time to confirmed clinically meaningful deterioration in hormonal treatment-related symptoms
- Notable adverse events:
- Hot flashes: More common in the enzalutamide + leuprolide and leuprolide-only arms (57–69% versus 22% for enzalutamide monotherapy)
- Gynecomastia nipple pain, and breast tenderness: More common with enzalutamide monotherapy
- Gynecomastia: 45% versus 8–9%
- Nipple pain: 15% versus 1–3%
- Breast tenderness: 14% versus 1%
Summary and Take-Home Messages
In this analysis of patient-reported outcomes from the EMBARK trial, the key take-home message is that the majority of trial patients maintained overall stable health-related quality-of-life outcomes throughout the study. However, there are notable differences between the study arms with respect to their relative impact on specific quality-of-life domains. A comprehensive understanding of these differences is key to engaging these patients in a shared decision-making process to strike a balance between maximizing oncologic benefits while maintaining quality-of-life measures that are most significant for the individual patients.Published October 2024


