Introduction
In November 2023, the United States Food and Drug Administration (FDA) approved enzalutamide, with or without concurrent leuprolide therapy, for conventional imaging-defined non-metastatic hormone-sensitive prostate cancer (M0 HSPC) patients with biochemical recurrence at high risk for metastasis.1 This drug approval followed the publication of the EMBARK trial, a randomized phase III trial of biochemically recurrent patients who had high-risk disease, defined by a PSA doubling time (PSADT) ≤9 months and a PSA level of ≥2 ng/mL above nadir following radiation therapy or ≥1 ng/mL after radical prostatectomy, with or without postoperative radiation therapy. Patients in this trial were randomized 1:1:1 to combination enzalutamide + leuprolide, leuprolide monotherapy, or enzalutamide monotherapy. This trial met its primary endpoint of improved 5-year metastasis-free survival with the combination of enzalutamide + leuprolide (87.3% versus 71.4% for leuprolide alone; HR 0.42, p < 0.001). Similarly, enzalutamide monotherapy was associated with superior 5-year metastasis-free survival rates, compared to leuprolide monotherapy (80% versus 71%; HR 0.63, p = 0.005).2
However, the ‘elephant in the room’ is that patients in the EMBARK trial, who were enrolled between January 2015 and August 2018, were staged using conventional imaging, as opposed to prostate-specific membrane antigen (PSMA) positron emission tomography (PET), which has emerged as a standard of care imaging modality for biochemically recurrent patients, owing to its improved sensitivity for detecting metastatic disease in this setting.3,4 As such, many experts in the field have suggested that there is a ‘discord’ between the study design of EMBARK and contemporary practice.
In this Center of Excellence article, we will discuss the results of the EMBARK trial within the context of contemporary PSMA PET imaging and discuss ongoing trials of biochemically recurrent M0 HSPC patients.
PSMA PET Findings in an ‘EMBARK-like’ Cohort
Had trial participants in EMBARK undergone a PSMA PET at study inclusion, what would a PSMA PET scan have shown? At ASCO 2023, Armstrong et al. presented the results of a post-hoc, retrospective analysis of four prospective studies of PSMA PET conducted at UCLA between 2016 and 2021 that included high-risk biochemically recurrent prostate cancer patients who would have met the EMBARK trial eligibility criteria. The study cohort included 183 patients, with a median time from primary therapy to PSMA PET of 39 months. The median serum PSA level at PSMA PET was 2.8 ng/mL, and the median PSADT was 3.6 months.
Overall, 85% of patients were PSMA PET positive, with an average of 3.9 lesions per case. Pelvic nodal disease was present in 56% of patients, and 30% had evidence of extra-pelvic nodal disease. Bone lesions were present in 26% of patients, and 4.4% of patients had visceral metastases:
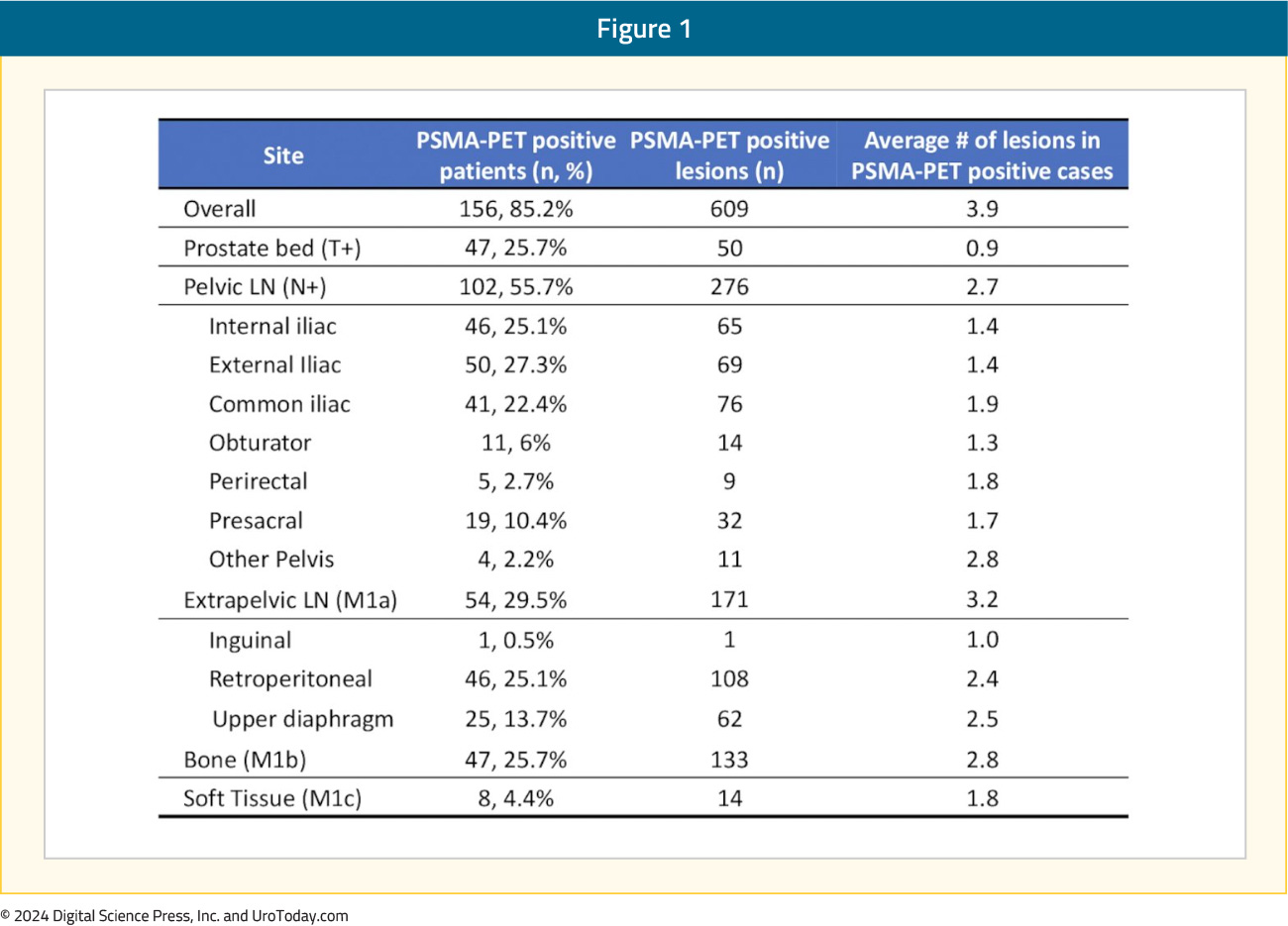
Overall, 23% of patients had evidence of nodal-only disease (i.e., TanyN1M0), 46% had distant metastases (TanyNanyM1), 37% of patients had oligometastatic disease, and 9% had polymetastatic disease (i.e., >5 M1 lesions).5 A visual distribution of the metastatic breakdown is shown below:
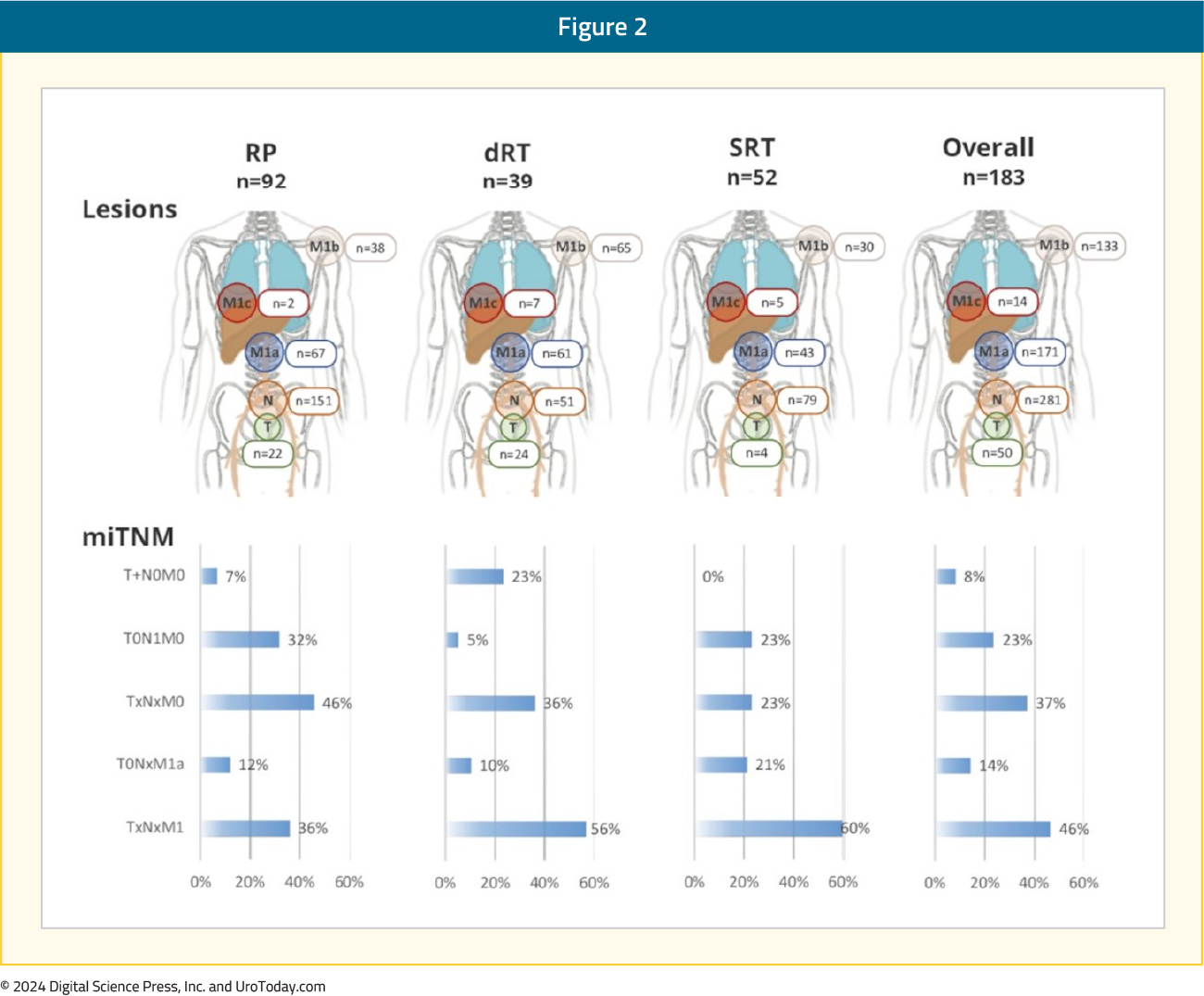
Metastasis-Directed Therapy or Systemic Therapy Intensification for ‘EMBARK-like’ Patients
One of the proposed treatment paradigms for the contemporary management of an ‘EMBARK-like’ patient is obtaining a PSMA PET scan and targeting PET-avid lesions with metastasis-directed therapy (MDT), particularly for patients with evidence of oligometastases (i.e., ≤5 lesions), to maximize the systemic therapy-free interval. There are, however, important differences between the EMBARK cohort and the three major phase II trials of MDT in oligorecurrent hormone sensitive prostate cancer: STOMP, ORIOLE, and EXTEND.6,7,8 The major difference is that patients in STOMP and ORIOLE had evidence of metastatic disease on non-PSMA PET imaging (STOMP: choline PET/CT; ORIOLE: conventional imaging [CT, MRI, and/or bone scan]), whereas those in EMBARK had no evidence of metastasis on conventional imaging.
It is important to note, however, that approximately 40% of patients of ‘EMBARK-like’ patients will have oligometastatic disease on a PSMA PET. There is evidence from the ORIOLE trial to support MDT targeting of all PSMA avid lesions. In this trial, pre-treatment 18F-DCFPyL-PET/CT was performed in all patients assigned to the MDT arm (n = 36), and the treating physicians and patients were blinded to the results of these scans. Sixteen patients (44%) had baseline PET-avid lesions that were not included in the MDT treatment fields and had significantly worse 6-month progression rates of 38% (95% CI 18.5–61.5%) compared to those without untreated lesions (5%; 95% CI 0–26.8%; p = 0.03). Furthermore, those with untreated sites of disease had higher rates of new metastases (per conventional imaging) at 6 months (62.5% versus 15.8%, p = 0.006) and worse median distant metastasis-free survival of 6 versus 29 months (HR: 0.19; 95% CI: 0.07–0.54, p < 0.001).6
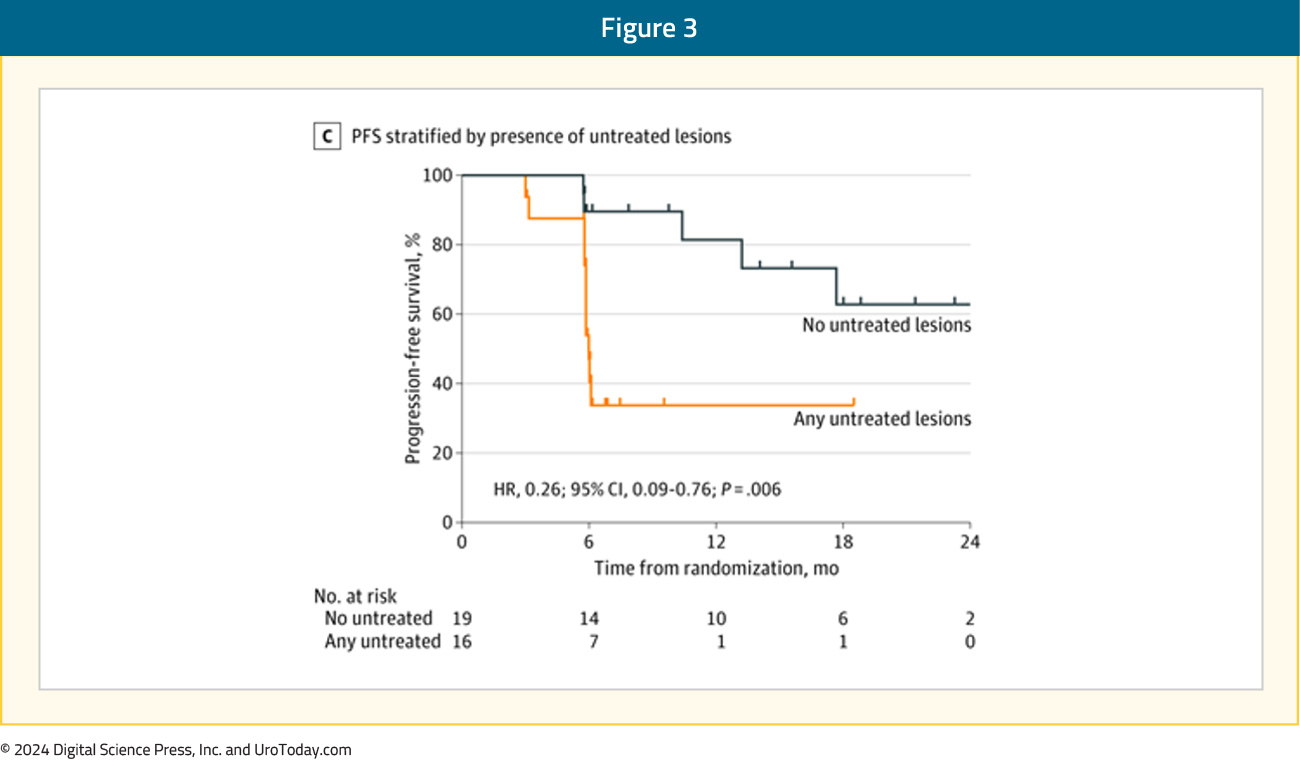
What about the use of MDT with systemic hormonal therapy? The phase II EXTEND trial demonstrated that the addition of MDT to 6 months of intermittent hormonal therapy significantly improved eugonadal progression-free survival (HR 0.32, p = 0.03). However, similar to the ORIOLE and STOMP, patients had evidence of metastatic disease, mostly identified on conventional imaging (75%). Additionally, approximately 90% of patients had a serum PSA level <2 ng/ml at study entry, whereas the median serum PSA level in EMBARK was 5–5.5 ng/ml. Notably, only 72% of the EXTEND trial patients had undergone definitive local therapy to the prostate, whereas all patients in EMBARK had undergone a radical prostatectomy and/or pelvic radiotherapy, with 50% having undergone both treatment modalities. As such, there are clear differences between the two study cohorts that limit trial comparisons and the generalizability of results and treatment approach used in EXTEND to EMBARK trial participants.
What about the use of MDT with androgen receptor pathway inhibitors? To date, there are no randomized trials comparing the added benefit of combining one modality with the other (ARTO trial of abiraterone +/- stereotactic body radiotherapy [SBRT] was in the castrate-resistant setting) in this disease space.9 The only published trial of combination MDT with androgen receptor pathway inhibitors in oligorecurrent metastatic prostate cancer patients is the phase II SATURN trial, in which patients received six months of leuprolide + abiraterone acetate/prednisone + apalutamide and SBRT to all metastases, with or without radiotherapy directed to the prostate bed and pelvic lymph nodes, after the first month of systemic therapy. Overall, 50% of patients maintained a PSA <0.05 ng/mL six months after testosterone recovery (primary endpoint), and the median eugonodal progression-free survival was 11.4 months.10
One pragmatic approach to treating an ‘EMBARK-like’ patient that has PSMA PET findings of metastatic disease (ie. conventional imaging negative, PSMA PET positive for metastatic disease) is to treat these patients with enzalutamide + leuprolide, rather than enzalutamide monotherapy or leuprolide monotherapy. The rationale for such an approach is that these patients are more “ENZAMET/ARCHES-like” patients, where there is a proven therapeutic benefit of enzalutamide + ADT versus ADT alone in the mHSPC setting.11,12
In summary, the only available level one evidence to inform the treatment of high-risk, biochemically recurrent M0 HSPC patients staged with conventional imaging comes from the EMBARK trial, where both enzalutamide combination therapy with leuprolide and enzalutamide monotherapy have demonstrated metastasis-free survival benefits. Future randomized trials in this space may provide further answers to the question of optimal management of these patients who have PSMA PET positive disease. For the time being, however, similar to the mHSPC treatment paradigm which relies on conventional imaging volume findings to dictate treatment recommendations, high-risk, biochemically recurrent patients with conventional imaging-defined M0 HSPC should be recommended to receive systemic therapy with androgen receptor pathway inhibitors.
Ongoing Trials Utilizing PSMA PET in the High-Risk, Biochemically Recurrent M0 HSPC Space
ARASTEPARASTEP (NCT05794906) is an ongoing randomized, phase III, double-blind, placebo-controlled trial of prostate cancer patients with high-risk biochemical recurrence, defined by a PSADT <12 months with PSA ≥ 0.2 ng/mL after primary radical prostatectomy (+/- adjuvant radiotherapy/salvage radiotherapy) or PSA ≥2 ng/mL above nadir after primary radiotherapy only, ≥1 PSMA PET/CT-positive lesion of prostate cancer without visible lesions on conventional imaging, and serum testosterone >150 ng/dL.
Approximately 750 patients from 184 sites worldwide will be randomized to oral darolutamide 600 mg twice daily or placebo, both with ADT, for 24 months, unless there is earlier evidence of disease progression, unacceptable toxicity, or any other withdrawal criteria are met. Of note, patients will suspend treatment after 24 months if serum PSA levels remain undetectable (i.e., <0.2 ng/mL). Patients whose PSA values remain detectable (i.e., ≥0.2 ng/mL) will continue with the study treatment until PSMA PET/CT progression. The trial design for ARASTEP is as follows:
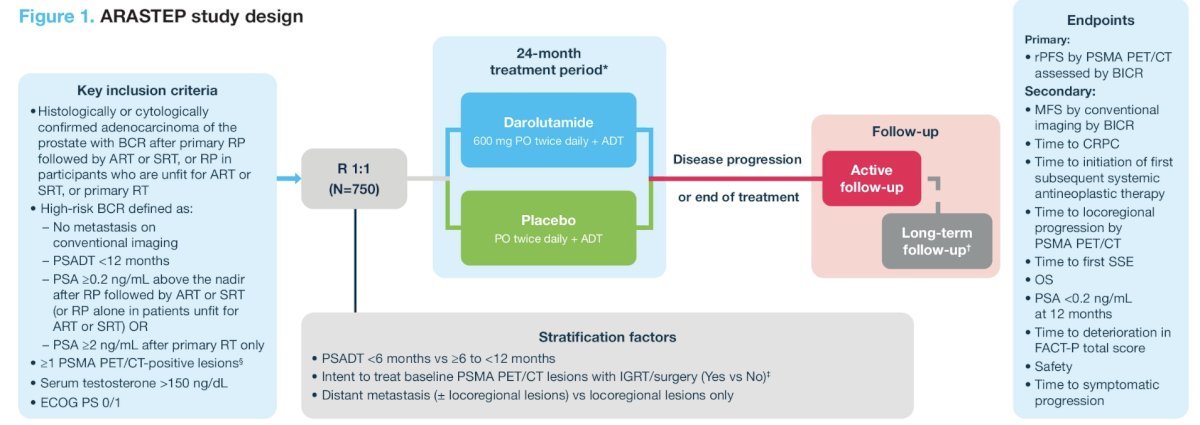
The primary study endpoint is radiographic progression-free survival by PSMA PET/CT, assessed by a blinded independent central review. Key secondary endpoints include:
- Metastasis-free survival by blinded independent central review
- Time to CRPC
- Overall survival
- Quality of life
- Safety
INDICATE (ECOG-ACRIN EA8191; NCT04423211) is a phase III trial that aims to evaluate the following in post-radical prostatectomy biochemically recurrent patients without evidence of metastases on conventional imaging:13
- Whether the addition of apalutamide to standard salvage radiotherapy plus short-term ADT improves survival in patients without extra-pelvic PET-detected lesions
- Whether the addition of MDT to the treatment intensification combination of salvage radiotherapy, short-term ADT, and apalutamide improves survival in patients with PET-detected lesions outside the pelvis
Eligible patients are those with biochemical recurrence post-radical prostatectomy, defined by a serum PSA level >0.5 ng/ml or >0.2 ng/ml, if detected within 12 months of the radical prostatectomy, and no evidence of extra-pelvic metastases on conventional imaging, who are candidates for standard of care salvage therapy (salvage radiotherapy to the prostate bed and pelvic node with concurrent short-term ADT). Of note, if a patient has a detectable PSA (≥0.01 ng/ml) at any time after radical prostatectomy and has a baseline PET that is positive for ≥1 lesion in any location, there is no minimum PSA requirement.
All patients will undergo baseline PET using one of the three FDA-approved tracers, at which point patients will be assigned to either Cohort 1 (PET negative for extra-pelvic metastases) or Cohort 2 (PET positive for extra-pelvic metastases). The study design is as follows:
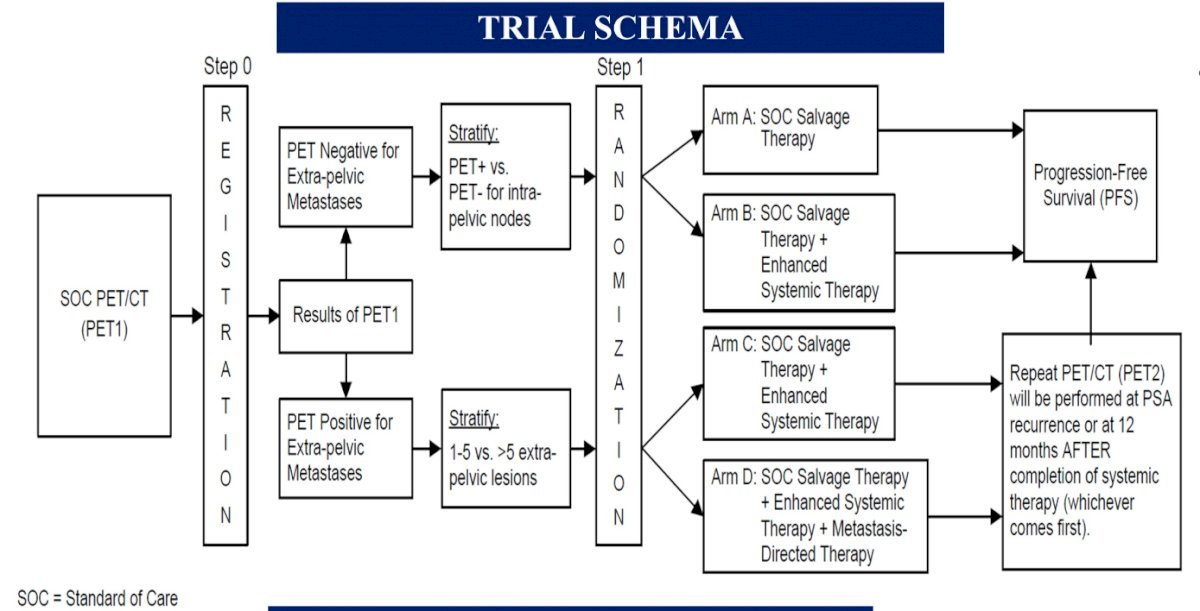
Patients in Cohort 1 (PET negative) will be randomized to either:
- Arm A: Salvage external beam radiotherapy (EBRT) + short-term ADT (leuprolide or goserelin) OR
- Arm B: Salvage EBRT + short-term ADT + apalutamide
Patients in Cohort 2 (PET positive for extra-pelvic metastases) will be randomized to either:
- Arm C: Salvage EBRT + short-term ADT + apalutamide OR
- Arm D: Salvage EBRT + short-term ADT + apalutamide + MDT (SBRT)
The primary endpoint in both cohorts is progression-free survival, defined as time from randomization to radiographic progression on conventional imaging, symptomatic disease progression, or death, whichever occurs first. Secondary endpoints include overall and event-free survival, toxicity, PET progression, and quality of life. The study accrual start date was October 2022, with an estimated primary completion date of December 2027.
Conclusions
Based on the current body of evidence, high-risk, biochemically recurrent patients without evidence of metastases on conventional imaging should be strongly considered for systemic therapy with enzalutamide combination therapy with leuprolide or monotherapy. Despite PSMA PET detecting extra-pelvic metastases in almost 50% of such patients, there is no high-level evidence, to date, to support the routine utilization of PSMA PET findings to direct treatment with SBRT. Ongoing trials will help address important remaining questions in this disease space, namely the value/utility of PSMA PET in patients with negative conventional imaging findings and the added benefit of PSMA PET-directed SBRT to systemic therapy intensification with androgen receptor pathway inhibitors in patients with PSMA PET/conventional imaging discordant findings.
Published November 2024


