Results from the PEACE-1 trial evaluating the role of prostate radiotherapy in patients with de novo, low-volume mHSPC were recently presented at ASCO 2023.2 These results add to the currently available data in this setting from both HORRAD and STAMPEDE Arm H.3-5 While all three trials include patients with de novo mHSPC, there are important between-trial differences in baseline cohort characteristics that affect the external validity/generalizability of the results and may explain the observed differences in the study outcomes.
In this Center of Excellence Article, we summarize the available evidence for prostate radiotherapy in the de novo mHSPC setting, focusing on those with low-volume disease per conventional imaging. We also highlight important differences between the three trials and discuss how clinicians may use these latest results to inform their clinical practice.
The Three Key Randomized Clinical Trials
HORRAD
HORRAD was a multicenter, prospective, randomized controlled trial of 432 patients with previously untreated, de novo mHSPC at 28 centers across The Netherlands between November 2004 and September 2014. All study-eligible patients had a PSA >20 ng/ml and documented bone metastases on bone scan. Patients were randomized in a 1:1 fashion to either ADT with external beam radiotherapy (EBRT) or ADT alone. ADT in both groups consisted of a luteinizing hormone-releasing hormone (LHRH) agonist plus a first-generation non-steroidal anti-androgen (e.g., bicalutamide) for four weeks as flare reduction. Patients in the EBRT group received either intensity-modulated or three-dimensional conformal radiotherapy to the prostate alone (70 Gy in 20 fractions or 57.76 Gy in 19 fractions), and pelvic lymph nodes were not included in the clinical target volume.
The median patient age in HORRAD was 67 years, and the median PSA was 142 ng/ml. At a median follow-up of 47 months, there were no significant differences in median overall survival between the two treatment arms: 45 and 43 months in the EBRT + ADT and ADT arms, respectively (HR: 0.90, 95% CI: 0.70-1.14, p=0.4):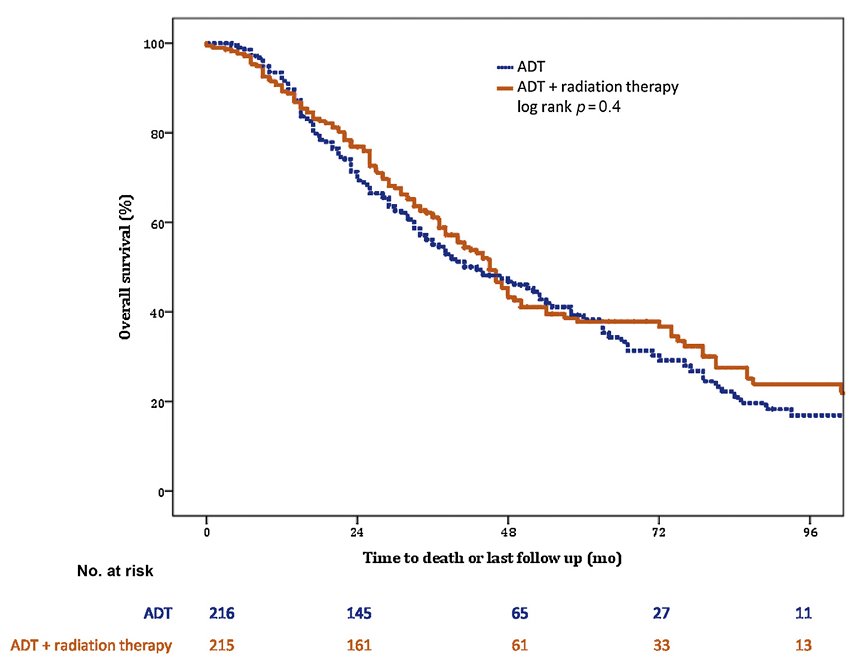
No subgroup analysis by CHAARTED volume criteria was performed, but subgroup analysis by a number of metastatic lesions suggested a potential overall survival benefit for radiotherapy in patients with <5 metastatic sites (HR: 0.68, 95% CI: 0.42-1.10):3
STAMPEDE (Arm H) was an open-label, randomized controlled phase III trial of 2,061 men from 117 hospitals across Switzerland and the UK. This arm randomized patients with de novo mHSPC in a 1:1 fashion to standard of care plus radiotherapy versus standard of care alone between January 2013 and September 2016. The standard of care was lifelong ADT with upfront docetaxel permitted from December 2015. Men allocated to radiotherapy received either a daily (55 Gy in 20 fractions over 4 weeks) or weekly (36 Gy in six fractions over 6 weeks) schedule that was nominated before randomization. The planning target volume consisted of the prostate only, the primary outcome was overall survival, and subgroup analysis by metastatic volume (CHAARTED criteria) was planned a priori.
The median patient age was 68.0 years, with a median PSA of 97 ng/ml; 18% of patients received early docetaxel. The radiotherapy schedule was almost equally split between the daily and weekly regimens. 54% of men had high metastatic burden compared to 40% with low metastatic burden (6% unknown). In the overall cohort, radiotherapy improved failure-free survival (HR 0.76, 95% CI 0.68–0.84) but not overall survival (HR 0.92, 95% CI 0.80–1.06) in the overall cohort. However, when stratified by metastatic burden, OS benefits were seen in the low volume group (HR 0.68, 95% CI 0.52-0.90) with restricted mean survival time improved by 3.6 months from 45.4 to 49.1.4
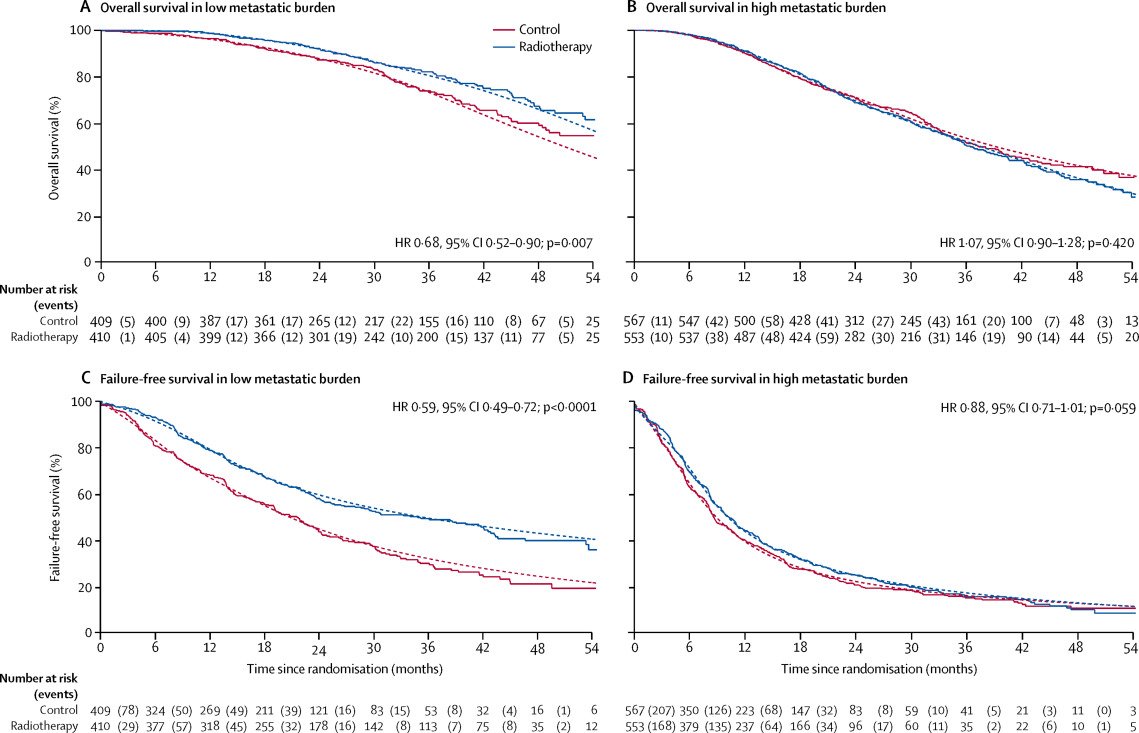
Updated results of this trial were published in 2022.5 With a median follow-up of 61.3 months, prostate radiotherapy continued to demonstrate overall survival benefits in patients with low metastatic burden (HR 0.64, 95% CI 0.52-0.79):
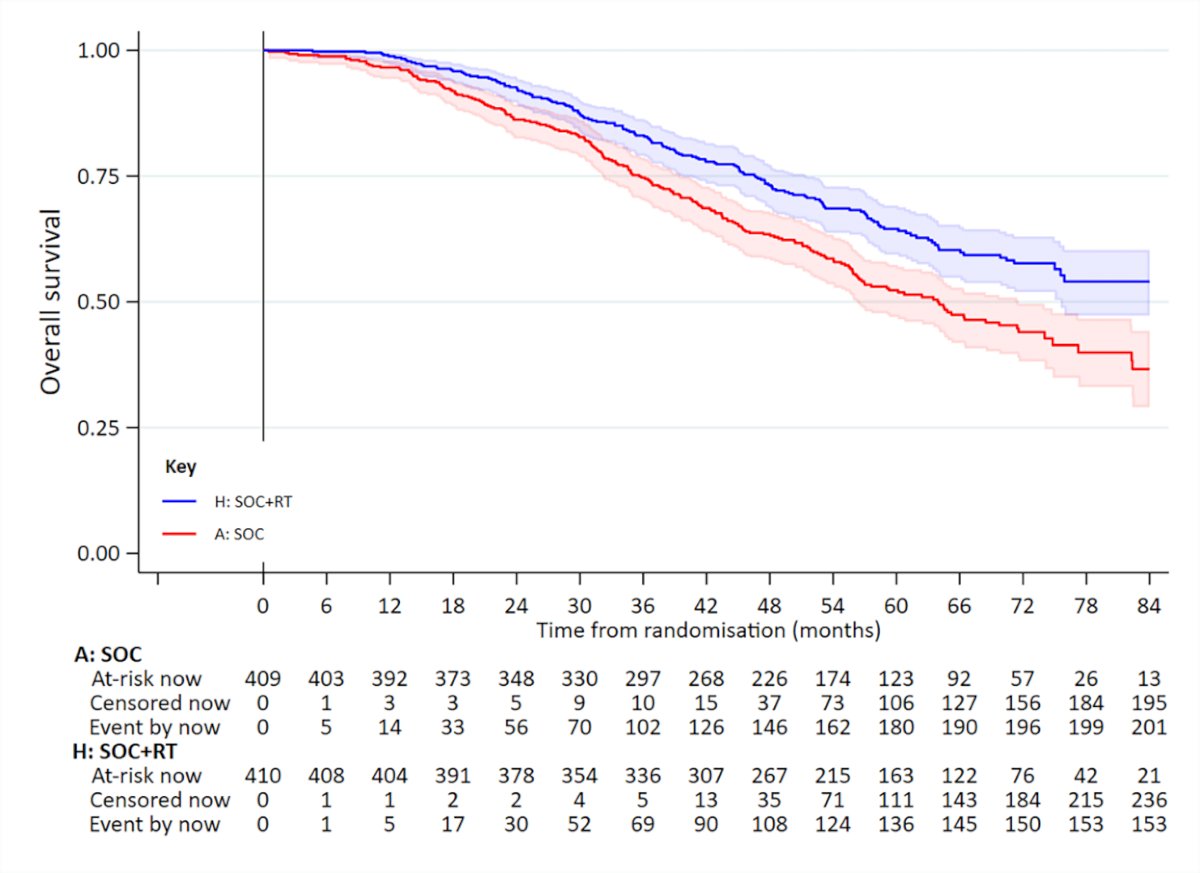
and no benefit was seen in patients with high metastatic burden (HR 1.11, 95% CI 0.96-1.28; interaction p=0.001). There were no significant between-group differences in:
- Time to symptomatic local events
- Global QoL or QLQ-30 Summary Score
- Long-term grade ≥3 urinary or bowel toxicity
The PEACE-1 trial included patients with de novo mHSPC, with eligible patients, allowed up to 3 months of ADT prior to randomization. This trial employed a two-by-two factorial design to assess, both separately and combined, the impact of abiraterone acetate/prednisone and prostate radiotherapy addition to standard of care therapy in men with de novo mHSPC. This trial randomized 1,173 patients in a 1:1:1:1 fashion to standard of care +/- abiraterone +/- radiotherapy: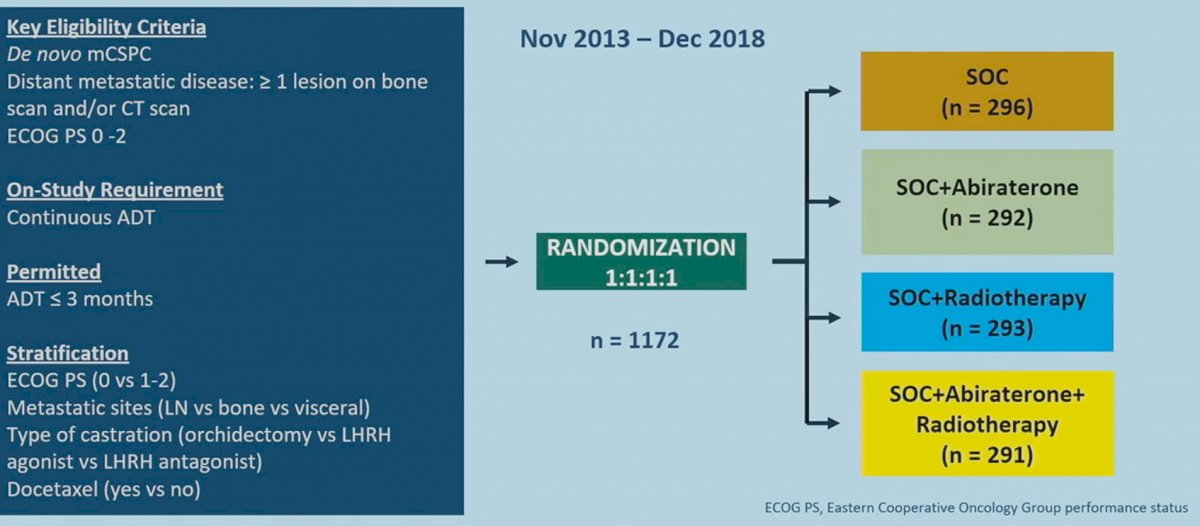
Standard of care treatment included continuous ADT or bilateral orchiectomy, with or without docetaxel at 75 mg/m2 every three weeks for six cycles. Various amendments were implemented during the trial due to the evolving standard of care in this disease space. After 2015, docetaxel was permitted as part of the standard of care per the investigator’s discretion and patient consent. After CHAARTED and STAMPEDE were reported,6,7 it became unethical to administer ADT alone, and so docetaxel administration was made mandatory.
Results of the PEACE-1 trial evaluating the addition of abiraterone acetate 1,000 mg/day + prednisone 5 mg twice daily to standard of care therapy have previously been published and have demonstrated that addition of abiraterone acetate/prednisone to standard of care: Abiraterone + ADT +/- docetaxel +/- radiotherapy was associated with rPFS (HR 0.54, 95% CI 0.41-0.71) and OS benefits (HR 0.82, 95% CI 0.69 – 0.98).8
At ASCO 2023, Dr. Alberto Bossi presented the results of the PEACE-1 trial assessing the efficacy and safety of prostate radiotherapy for patients with low volume, de novo mHSPC. Radiotherapy to the prostate was delivered in 37 fractions for a cumulative dose of 74 Gy, and pelvic lymph nodes were not included. The co-primary endpoints for this analysis were overall survival and rPFS, assessed per the Prostate Cancer Working Group 2 (PCWG2) criteria.
The median age in the low-volume cohort was 67 years. The median PSA was 9.0 and 10.3 ng/ml in the standard of care + radiotherapy and standard of care arms, respectively. Notably, 50% of patients received docetaxel as standard of care treatment, with 50% of the cohort overall randomized to receive additional abiraterone acetate/prednisone. For the rPFS outcome, a qualitative interaction between radiotherapy and abiraterone was observed (p=0.026), and, given this, each of the four experimental arms were assessed individually. Conversely, for overall survival, the pre-defined threshold for statistical interaction was not reached (p=0.12), and thus the two radiotherapy arms (radiotherapy + standard of care and radiotherapy + standard of care + abiraterone) were pooled for this analysis.
The addition of prostate radiotherapy to standard of care + abiraterone was associated with a significant rPFS benefit (median 7.5 versus 4.4 years, p=0.02). However, the addition of radiotherapy to standard of care alone was not associated with a rPFS benefit (median 2.6 versus 3.0 years; HR 1.11, 95% CI 0.67 – 1.84):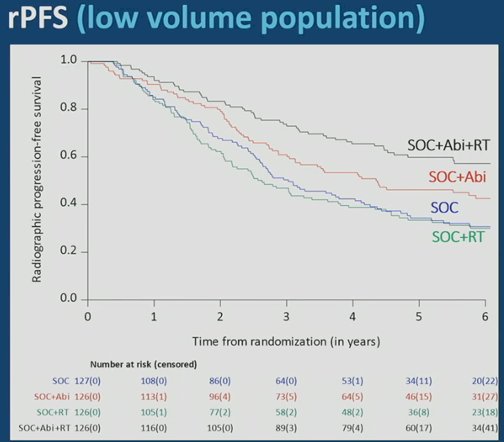
The addition of prostate radiotherapy to standard of care +/- abiraterone was associated with significant improvements in time to castration resistance in the low-volume cohort (median 3.4 versus 2.5 years; HR 0.74, 95% CI 0.60 – 0.92).
With regards to overall survival, the addition of prostate radiotherapy to either standard of care alone or standard of care + abiraterone was not associated with significant benefit. In the standard of care + abiraterone arm, the addition of prostate radiotherapy was associated with modest, non-significant overall survival benefits (HR 0.77, 95% CI 0.51 – 1.16). Similarly, addition of prostate radiotherapy to standard of care alone did not improve overall survival (HR 1.18, 95% CI 0.81- 1.71):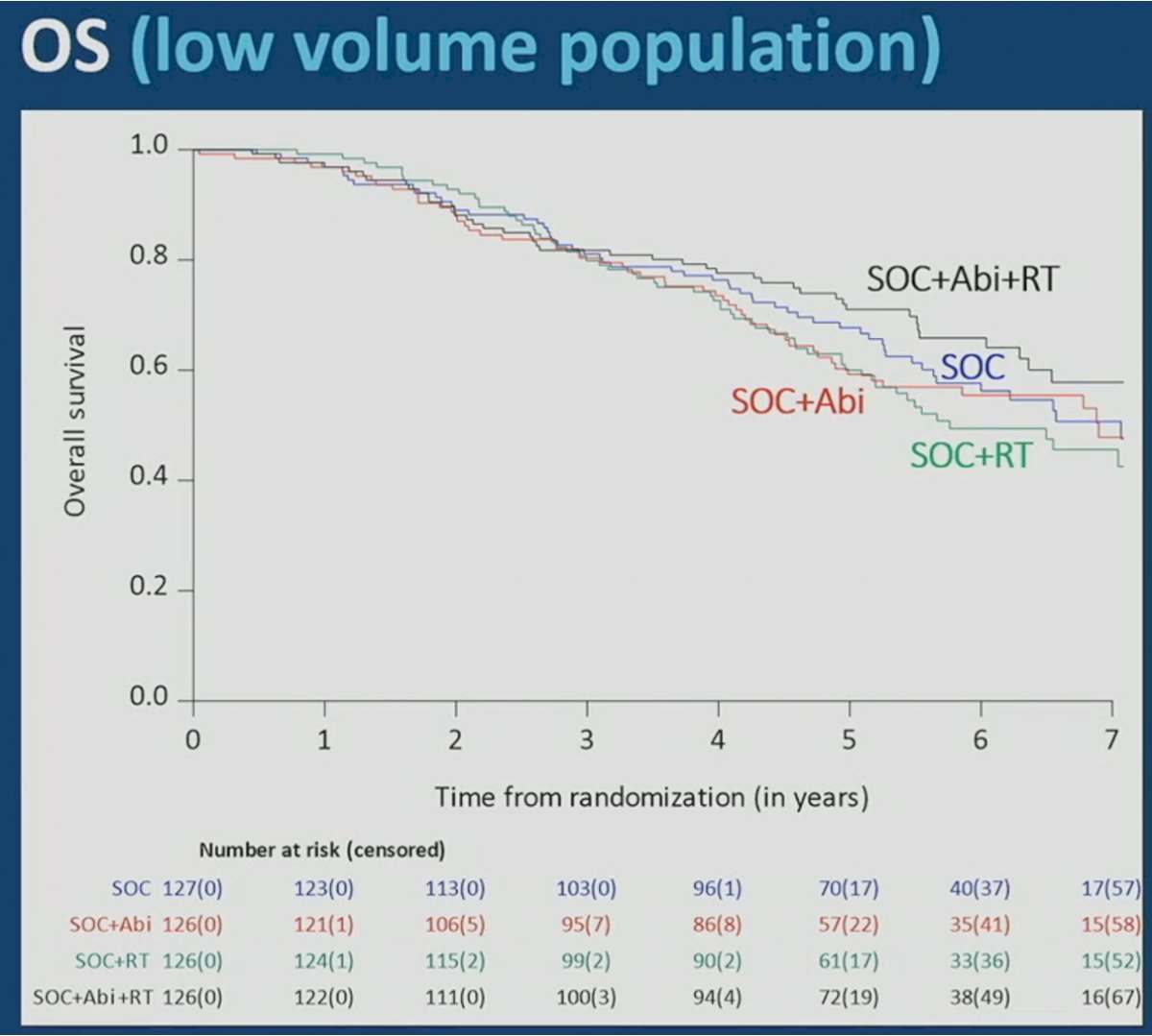
One of the common concerns regarding early treatment intensification is the additive toxicity and adverse effects on patient quality of life. Interestingly, the addition of prostate radiotherapy to standard of care +/- abiraterone was associated with significant improvements in time to serious genitourinary events in the low volume cohort. Overall, a similar toxicity profile was observed among patients receiving radiotherapy versus not, with the most common grade 3-5 adverse events as follows:
- Hypertension (23% and 18% in the radiotherapy and no radiotherapy arms, respectively)
- Neutropenia (5% and 7%)
- Hepatotoxicity (3% and 4%)
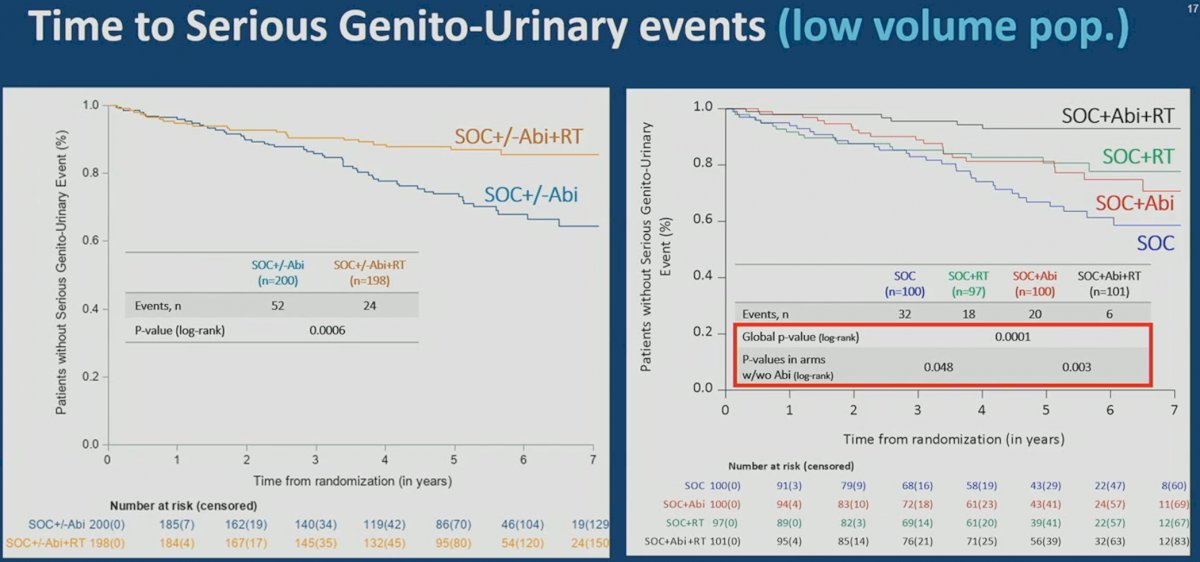
Examining Differences Between the Trials and Implementing Results into Clinical Practice
As follows is a table comparing the three randomized trials evaluating prostate radiotherapy in the de novo mHSPC setting:
|
Study |
HORRAD |
STAMPEDE Arm H |
PEACE-1 |
|
Number of patients |
432 |
2,061 |
1,173 |
|
Patient age, median |
67 |
68 |
67 |
|
Baseline PSA, median |
142 |
97 |
9.0 – 10.3 |
|
Prior treatment with docetaxel |
0% |
18% |
50% |
|
Prior treatment with an ARST |
0% |
0% |
50% (abiraterone) |
|
Prostate radiotherapy dose and schedule |
70 Gy/20 fx 57.76 Gy/19 fx |
Daily: 55 Gy/20 fx Weekly: 36 Gy/6 fx |
74 Gy/37 fx |
|
Inclusion of pelvic lymph nodes |
No |
No |
No |
|
Overall survival outcomes |
Overall cohort: HR=0.90, 95% CI: 0.70-1.14
<5 bone metastases: HR=0.68, 95% CI: 0.42-1.10
|
Overall cohort: HR=0.92, 95% CI: 0.80-1.06
CHAARTED low volume: HR=0.64, 95% CI: 0.52-0.79 |
CHAARTED low volume only:
-SOC + abiraterone arm: HR=0.77, 95% CI: 0.51-1.16
-SOC arm: HR=1.18, 95% CI: 0.81-1.71 |
ARSI: Androgen receptor signaling inhibitor
PSA: Prostate-specific antigen
While all three trials evaluated the role of prostate radiotherapy in the de novo mHSPC setting, there are important differences in the study cohort characteristics that may explain why overall survival benefits were observed in the low-volume patients in HORRAD and STAMPEDE Arm H, but not PEACE-1.
PEACE-1 patients represent a more heavily pre-treated cohort of patients, compared to those from the other two trials. In this trial, 50% of patients had received each of docetaxel and abiraterone, whereas only 18% of patients in STAMPEDE received docetaxel (none received an ARSI) and none of the patients in HORRAD received doublet/triplet systemic therapy: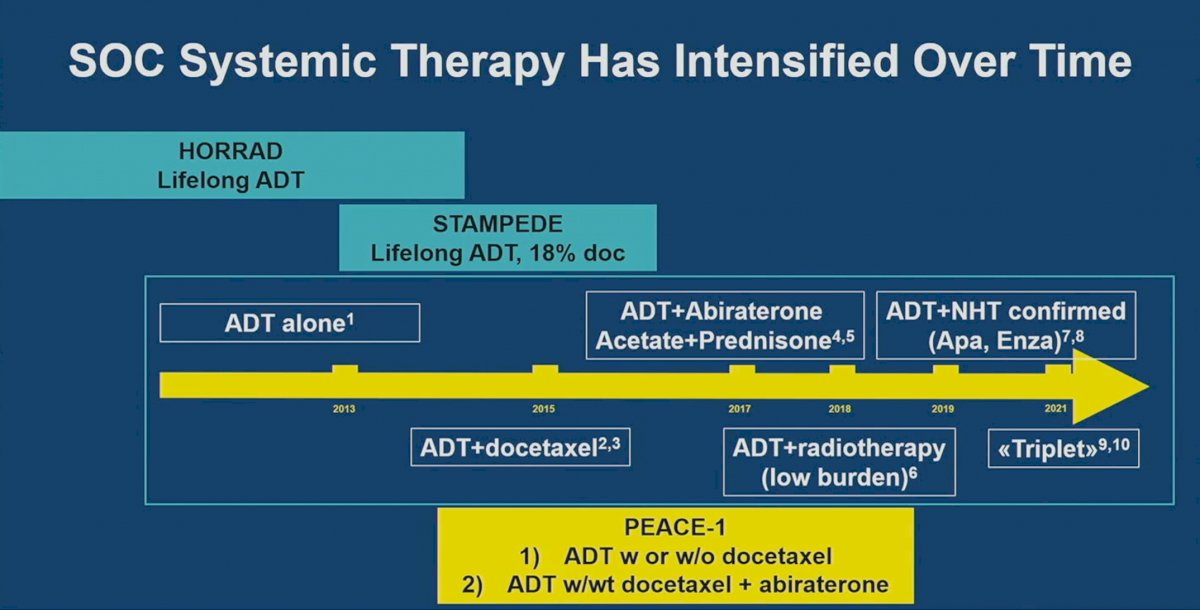
It has been argued that the benefit of local therapy on survival depends on the effectiveness of systemic therapy. While this remains an empiric concept, the underlying rationale here is that there may be a “sweet spot” for radiotherapy in mHSPC patients. This concept is depicted in the figure below. Based on the patient's disease characteristics and systemic treatment received, it is likely that HORRAD and STAMPEDE fall within that “sweet spot” of where the benefit of local therapy on survival is optimal. Conversely, administering radiotherapy in a more advanced, heavily pre-treated setting (i.e., along the downward slope of this curve) may prove to be less effective (PEACE-1):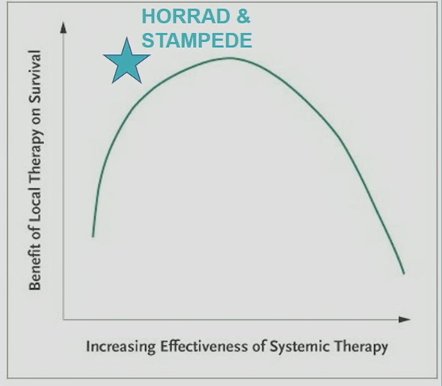

If the observed between-trial differences in overall survival benefits with radiotherapy were due to the PEACE-1 trial patients being more heavily pre-treated, then why were no overall survival benefits observed in the PEACE-1 standard of care arm? In other words, why did the results observe in this arm (HR 1.18, 95% CI 0.81 – 1.71) differ from those in STAMPEDE Arm H (HR 0.64, 95% CI 0.52 – 0.79)?
Potential reasons include the following:
- 50% of patients in the PEACE-1 standard of care arm received docetaxel compared to only 18% in STAMPEDE Arm H
- The emergence of more effective next-line systemic therapies over the past decade may attenuate the benefits of prostate radiotherapy in this setting. This is reflected in a median overall survival of 83 months in the PEACE-1 standard of care arm, compared to 64 months in STAMPEDE standard of care arm
In light of the recent findings from the PEACE-1 trial, should we continue to offer prostate radiotherapy to patients with de novo, low-volume mHSPC? While no overall survival benefits were observed in either the standard of care or standard of care + abiraterone arms, there are compelling reasons to continue to offer radiotherapy in this setting:
- Low volume mHSPC patients receiving radiotherapy had significantly prolonged time to development of castration resistance
- Patients in the abiraterone + standard of care arm had significantly prolonged rPFS. Given that current guidelines recommended administering both an ADT + an ARSI as part of doublet or triplet therapy regimens,1 this arm likely represents a more contemporary cohort of patients reflecting current clinical practice.
- Prostate radiotherapy may improve time to serious genitourinary events
Conclusion
Results from the PEACE-1 trial call into question the clinical efficacy of prostate radiotherapy for patients with de novo, low-volume mHSPC. While no overall survival benefits were observed in this trial, potentially due to underlying differences in baseline patient characteristics compared to HORRAD and STAMPEDE Arm H, rPFS improvement among those receiving concurrent abiraterone, prolonged time to castration resistance in all low-volume patients, along with improvements in time to serious genitourinary events need to be considered when assessing patients for candidacy of prostate radiotherapy in clinical practice. Currently, shared decision making between the oncology team, the patient, and their families should be undertaken when deciding on the utility of prostate radiotherapy for low volume mHSPC patients. Future trials in this disease space will also evaluate the role of radical prostatectomy for patients with de novo mHSPC.
Written by:
- Rashid Sayyid, MD, MSc, Urologic Oncology Fellow, Division of Urology, University of Toronto, Toronto, Ontario
- Zachary Klaassen, MD, MSc, Urologic Oncologist, Assistant Professor Surgery/Urology at the Medical College of Georgia at Augusta University, Georgia Cancer Center, Augusta, GA


