
The incidence of metastatic prostate cancer at diagnosis ranges from ~5-50%, with significant geographic differences as previously described.2 Such patients are defined as having de novo or synchronous mHSPC. Additionally, there exits a subset of men initially diagnosed with non-metastatic disease, many of whom have received prior definitive local treatment, who will have progression to a metastatic state prior to development of castration resistance. This is known as metachronous mHSPC. This distinction between synchronous (i.e. de novo) and metachronous presentations is of utmost clinical importance given the known differences in underlying genomic mutational profiles and prognoses, influencing the subsequent choice of treatment intensification.3,4 These two cohorts can be further subdivided based on the volume of metastatic disease at presentation: low and high volumes. The CHAARTED high-volume criteria have been widely adopted in clinical practice, with high volume patients defined as follows: presence of visceral metastases or ≥4 bone lesions with ≥1 beyond the vertebral bodies and pelvis.5
As such four distinct subgroups become clinically relevant (median overall survival per CHAARTED and GETUG-15 among men receiving ADT alone, ie. the control groups in these trials):
- Synchronous and high volume: 3 years
- Synchronous and low volume: 4.5 year
- Metachronous and high volume: 4.5 years
- Metachronous and low volume: ~8 years
Index Patient 2: Synchronous Low Volume mHSPC
Case presentation: A 65-year-old male presents to his primary care physician to establish care. His older brother had recently been diagnosed with prostate cancer, which prompted this visit. The patient had not previously been screened for prostate cancer. A digital rectal exam was performed, with evidence of asymmetric left prostatic enlargement and firmness. A serum PSA test was obtained, which demonstrated a PSA level of 28.3 ng/ml. The patient was referred to a urologist, who performed a systemic 12-core prostate biopsy demonstrating evidence of Gleason Score 9 (4+5) disease involving 2/12 cores, with 3 other cores harboring Gleason Score 8 (4+4) prostate cancer. A staging work up was ordered with a CT abdomen/pelvis demonstrating bilateral pelvic lymph node enlargement (1.5 cm in largest diameter) and a bone scan confirming the presence a right iliac crest osteoblastic lesion. There was otherwise no evidence of other bone metastases or visceral organ involvement. A chest CT scan was ordered and was similarly negative. Routine blood work up was a complete blood count and a comprehensive metabolic panel was negative.Doublet Therapy: ARATs + ADT
There is consistent evidence across all major published phase III trials to support an overall survival benefit to the addition of an ARAT to ADT in patients with low-volume disease. This is reflected, as follows:
- LATITUDE (abiraterone + ADT versus ADT alone; all de novo): HR 0.72; 95% CI: 0.47 to 1.109
- STAMPEDE (abiraterone + ADT versus ADT alone; >90% de novo): HR: 0.64; (95% CI: 0.42 to 0.96)10
- TITAN (apalutamide + ADT versus ADT alone; 10% prior docetaxel): HR: 0.52; 95% CI: 0.35 to 0.7911
- ENZAMET (enzalutamide + ADT +/- docetaxel versus non-steroidal antiandrogen + ADT +/- docetaxel): HR : 0.54; 95% CI: 0.39 to 0.748
- ARCHES (enzalutamide + ADT versus ADT alone; 18% prior docetaxel): HR: 0.66; 95% CI: 0.43 to 1.0312
Docetaxel in Synchronous, Low Volume mHSPC
At ASCO 2022, the results of a STOPCAP M1 collaborative meta-analysis of individual patient data from GETUG-15, STAMPEDE, and CHAARTED was presented. This meta-analysis included all 2,261 randomized patients, with median follow-up of 6 years. There were clear benefits for docetaxel on overall survival (HR: 0.79, 95%: CI 0.70 to 0.88), progression-free survival (HR: 0.70, 95% CI: 0.63 to 0.77) and failure-free survival (HR: 0.64, 95% CI: 0.58 to 0.71) in the overall pooled cohort. With evidence of non-proportional hazards, the estimated 5-year absolute differences were: overall survival 11% (95%: CI 6 to 15%), progression-free survival 9% (95% CI: 5 to 13%) and failure-free survival 9% (95% CI: 6 to 12%).
Subgroup analysis however demonstrated a non-significant benefit to docetaxel addition in patients with synchronous, low-volume prostate cancer (HR: 0.86; 95% CI 0.68 to 1.08).
As such, these results do not support the routine use docetaxel in the first line setting for the majority of patients with synchronous, low volume mHSPC. Chemo-fit patients with higher volume of disease not meeting the CHAARTED high-risk criteria however may still benefit from such treatment in select clinical scenarios.
Primary Radiotherapy for synchronous, low volume mHSPC patients
Beyond systemic treatment intensification, local prostate-directed therapy may allow for local treatment intensification. While a surgical approach using radical prostatectomy has been described, high quality data are limited to radiotherapy. Of note, the SWOG 1802 trial is accruing patients with a surgical arm in the setting of mHSPC to further assess the impact of cytoreductive prostatectomy in this disease space.
In addition to the PEACE-1 trial that will assess local RT in a 2:2 factorial design in patients treated with standard of care + docetaxel + ADT with or without abiraterone, there are two trials to date that have evaluated the role of local radiotherapy to the prostate in patients with mHSPC.
STAMPEDE ARM H
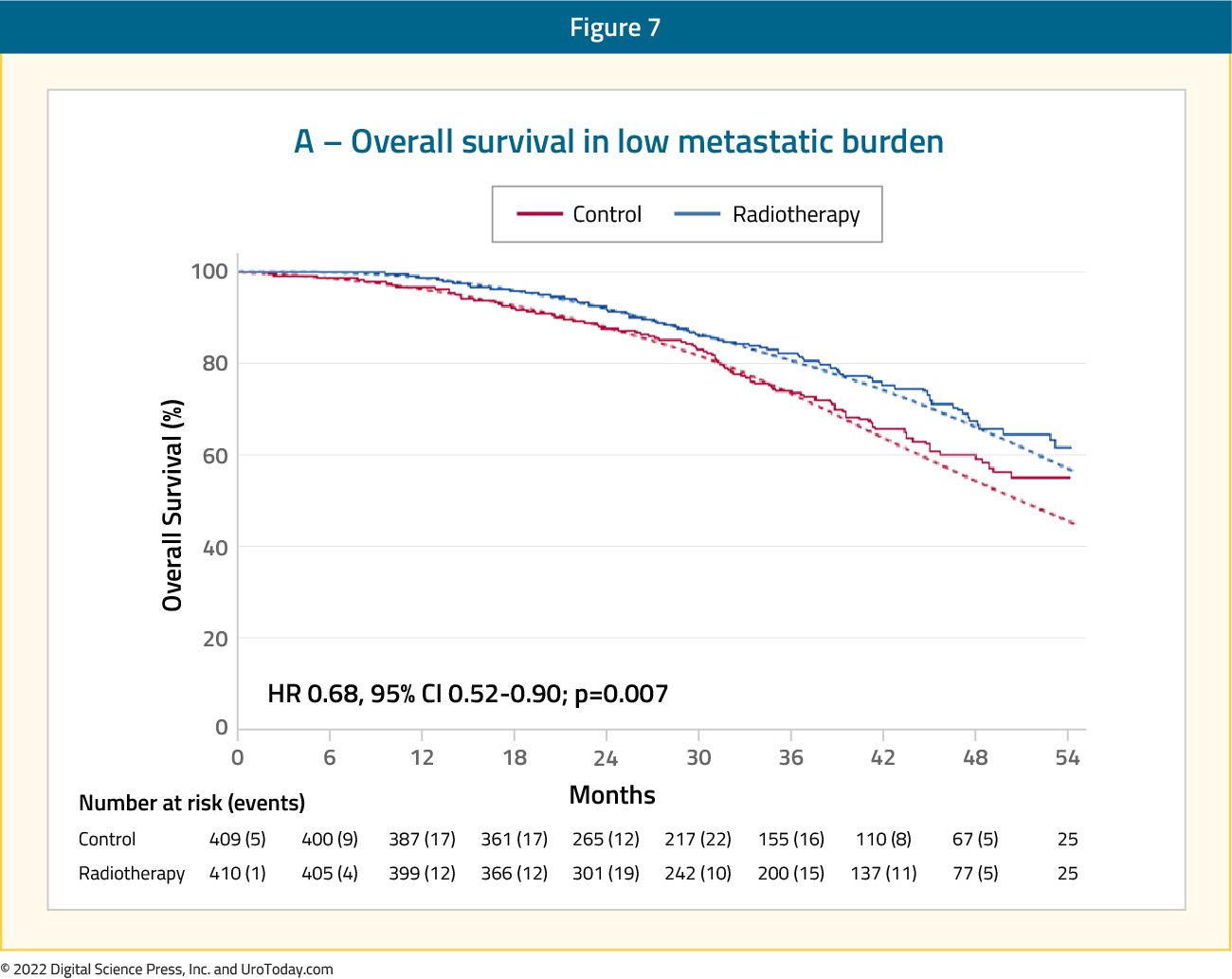
STAMPEDE (Arm H) was an open label, randomized controlled phase III trial of 2,061 men at 117 hospitals across Switzerland and the UK. This arm randomized patients with de novo mHSPC in a 1:1 fashion to standard of care + radiotherapy or standard of care alone between January 2013 and September 2016. Standard of care was defined as lifelong ADT with upfront docetaxel permitted from December 2015 onwards. Men allocated to radiotherapy received either a daily (55 Gy in 20 fractions over 4 weeks) or weekly (36 Gy in six fractions over 6 weeks) schedule that was nominated before randomization. The primary outcome for this trial was OS. Subgroup analysis by metastatic volume (CHAARTED criteria) was planned a priori.
Median follow up for STAMPEDE Arm H was 37 months, median patient age was 68.0 years, and median PSA was 97 ng/ml. There were 18% of patients that received early docetaxel. In the overall cohort, radiotherapy improved failure-free survival (HR: 0.76, 95% CI: 0.68 to 0.84) but not overall survival (HR: 0.92, 95% CI: 0.80 to 1.06) in the overall cohort. However, when stratified by metastatic burden, overall survival benefits were seen in the low volume group (HR: 0.68, 95% CI: 0.52 to 0.90) with restricted mean survival time improved by 3.6 months from 45.4 to 49.1:13
HORRAD
HORRAD was a multicenter prospective randomized clinical trial of 432 patients with previously untreated, de novo mHSPC at 28 centers across The Netherlands between November 2004 and September 2014. All eligible patients had a PSA >20 ng/ml and documented bone metastases on bone scan. Patients were randomized in a 1:1 fashion to either ADT with EBRT or ADT alone, with a primary endpoint of overall survival. The median PSA was 142 ng/mL and over a median follow up of 47 months, the median OS was non-significantly different at 45 months in the radiotherapy + ADT arm compared to 43 months in ADT alone arm (HR 0.90, 95% CI: 0.70 to 1.14).14
In summary, it appears that the majority of patients with synchronous, low volume mHSPC benefit from early systemic treatment intensification with ARAT addition to ADT. Addition of docetaxel may be considered in select patients such as those with extensive lymphadenopathy; however, evidence to support such practice remains unclear. Furthermore, such patients should be offered primary radiotherapy to the prostate gland in the appropriate clinical settings.
Written by:
- Rashid K. Sayyid, MD, MSc, University of Toronto, Toronto, Ontario
- Zachary Klaassen, MD, MSc, Medical College of Georgia, Augusta, Georgia, USA


