Use of Indwelling Urinary Catheters | Acute Care Catheter Use | Indications for indwelling urinary catheter use
Selected peri-operative needs | LTC Catheter Use | References
Each year, urinary catheters are inserted in more than 5 million patients in acute care hospitals and long-term care (LTC) facilities. Historically, indwelling urinary catheters (IUC) have been used in the chronically, medically compromised older adults.
The settings in which the prevalence of long-term IUCs usage is the greatest are: 1) skilled nursing facilities, where they are used in residents with UI, and 2) homes where the person requires skilled nursing visits.
In the home-care setting, the prevalence of IUCs is growing with the increasing number of older adults. The median time of indwelling catheter use in home care is reported as 3 to 4 years. However, the number of “home-bound” patients who use a catheter indefinitely to manage UI or because of urinary retention has not been well documented in medical or nursing research.
Indications for Indwelling Catheter (medical necessity)
Indwelling catheter overuse occurs when a device is in place without an appropriate indication. There are two ways of reducing IUC use: 1) by minimizing the initial placement of IUCs and 2) by reducing the duration of each catheterization. Urinary catheters have various medical indications but the most common is short term drainage of the urinary bladder. For some patients with upper tract deterioration due to elevated bladder storage pressures (e.g. poor compliance from prior radiation therapy, neurogenic disease, etc.), an IUC may have a role. The catheter permits low pressure, unimpeded drainage of urine from the upper urinary tract through the bladder and then directly into a collection receptacle. The following are indications for IUC use.
- Short term for acute urinary retention:
- Sudden and complete inability to void
- Need for immediate and rapid bladder decompression
- Monitoring of intake and output
- Temporary relief of bladder outlet obstruction secondary to:
- Enlarged prostate gland in men
- Urethral stricture
- Obstructing pelvic organ prolapse in women
- Chronic urethral obstruction or urinary retention and surgical interventions, or the use of intermittent catheterization, has failed or is not feasible, or both
- Short term following a urological or gynecological surgical procedure
- Irreversible medical conditions are present (e.g., metastatic terminal disease, coma, end stages, of other conditions)
- Presence of stage III or IV pressure ulcers that are not healing because of continual urine leakage
- Instances in which a caregiver is not present to provide incontinence care
Use of Indwelling Urinary Catheters
Although indwelling urinary catheters are commonly used in most clinical settings, data suggest that more than 20% of these catheters are placed without a specific medical indication and that they often remain in place without the knowledge of the patient’s physician. Studies of the appropriateness of use of urinary catheters indicate that 21 to 38% of initial urinary catheterizations are unjustified, and one-third to one-half of days of continued catheterization are unjustified. The current challenges are to develop effective methods to sensitize the minds of clinicians to avoid the routine use of indwelling catheters, remove catheters when they are no longer needed, develop alternative methods for care of urinary incontinence (UI), employ noninvasive methods to measure bladder function and urine output, and improve urine drainage systems.
Catheter Use in Acute Care Setting (Hospitals, Acute Rehabilitation)

In acute care hospital settings, approximately 12-16% of adult patients and up to 25% of all hospitalized patients usually for surgery, urine output measurement, urinary retention, or UI. Their use is greater in high acuity patient units, with critical care and intensive care units having the highest. At least 8%-23% of patients admitted through the emergency room have an IUC. Nearly 50% of surgical patients remain catheterized beyond 48 hours postoperatively; approximately 50% of medical patients do not have a clear indication for an IUC.
Hospitals use IUCs more than any other medical device. Because the most important risk factor for catheter-associated bacteriuria is duration of catheterization, most catheters in hospitalized patients are placed for only 2 to 4 days.
Extended indwelling catheter use in older adult patients sustaining hip fracture who are discharged to skilled nursing facilities with a catheter in place have been associated with poorer outcomes because these individuals are at higher risk of rehospitalization for CAUTIs and sepsis. Increased mortality at 30 days is seen in these individuals when compared to patients whose catheter was removed prior to discharge. In hospitalized older medical patients with UI, without a specific indication, an IUC has been associated with a greater risk of death - four times as great during hospitalization and two times as great within 90 days after discharge.
The risk of infection is associated with the method and duration of catheterization, the quality of catheter care, and host susceptibility. Around 50% of hospitalized patients catheterized longer than 7 to 10 days contract bacteriuria.
Although frequently asymptomatic, 20 to 30% of individuals with catheter-associated bacteriuria will develop symptoms of CAUTI. Many of these infections are serious and lead to significant morbidity and mortality.
Catheter Use in a Nursing Home
The prevalence of indwelling urinary catheter use in nursing homes has been established as 5-7%.
It may be greater in facilities that have poor success with toileting programs because the catheter is used as a means to maintain resident dryness.
At least 40% of all infections seen in nursing homes are in the urinary tract. Of these infections, 80% are due to urinary tract catheterization and instrumentation.
CAUTI is of major importance because of its effect on outcomes and treatment costs. The major reason for use of an indwelling catheter in LTC is incontinence or for healing a pressure injury.
Catheter Use in Home Care
In the community, the prevalence of IUC is difficult to determine as many of the long-term IUC patients are lost to urologic follow-up and are managed by home care nurses or allied clinicians. A National Home and Hospice Care Survey in 2007 reported catheter prevalence in home care (excluding hospice) at 9% (n = 4683) or 135,000 people with catheters of the 1.5 million home care patients in 2007. (http://www.cdc.gov/nchs/fastats/homehealthcare.htm).
Alternatives to indwelling urinary catheter use
1. Before placing an indwelling catheter, please consider if these alternatives would be more appropriate:
- Bedside commode, urinal, or continence garments: to manage incontinence.
- Bladder management through the use of a bladder scanner: to assess and confirm urinary retention, prior to placing catheter to release urine.
- Straight catheterization: for one-time, intermittent, or chronic voiding needs.
- External “condom” catheter: appropriate for cooperative men without urinary retention or obstruction.
2. Before placing an indwelling catheter, does the patient have one of the following appropriate indications* for placing indwelling urinary catheters?
- Acute urinary retention: e.g., due to medication (anesthesia, opioids, paralytics), or nerve injury
- Acute bladder outlet obstruction: e.g., due to severe prostate enlargement, blood clots, or urethral compression
- Need for accurate measurements of urinary output in the critically ill
- To assist in healing of open sacral or perineal wounds in incontinent patients
- To improve comfort for end of life, if needed
- Patient requires strict prolonged immobilization (e.g., potentially unstable thoracic or lumbar spine, multiple traumatic injuries such as pelvic fracture)
Selected peri-operative needs:
- Urologic surgery or other surgery on contiguous (adjacent) structures of the genitourinary tract
- Anticipated prolonged duration of surgery (Note: catheters placed for this reason should be removed in PACU)
- Large volume infusions or diuretics anticipated during surgery
- Need for intraoperative monitoring of urinary output
References
1. Centers for Medicare & Medicaid Services. Nursing Home Data Compendium. 2013. http://www.cms.gov/Medicare/Provider-Enrollment-andCertification/CertificationandComplianc /downloads/nursinghomedatacompendium_508.pdf.
2. Fakih MG, Heavens M, Ratcliffe CJ, Hendrich A. First step to reducing infection risk as a system: evaluation of infection prevention processes for 71 hospitals. Am J Infect Control. 2013;41:950-54. doi:10.1016/j.ajic.2013.04.019
3. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, HICPAC. Guideline for prevention of catheter associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31:319-26. doi: 10.1086/651091.
4. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter‐associated urinary tract infections 2009. Infect Control Hosp Epidemiol;31:319‐26.
5. Holroyd-Leduc JM, Sen S, Bertenthal D, Sands LP, Palmer RM, Kresevic DM, ………. Landefeld CS. The relationship of indwelling urinary catheters to death, length of hospital stay, functional decline, and nursing home admission in hospitalized older medical patients. Journal of the American Geriatrics Society, 2007;55;227–233.
6. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75.
7. Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109(6):476-480.
8. Saint S, Kowalski CP, Kaufman SR, et al. Preventing hospital‐acquired urinary tract infection in the United States: a national study. Clin Infect Dis 2008;46:243‐50.
9. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54:1055‐61.
10. Schuur JD, Chambers JG, Hou PC. Urinary catheter use and appropriateness in U.S. emergency departments, 1995-2010. Acad Emerg Med. 2014 Mar;21(3):292-300. doi: 10.1111/acem.12334Urinary catheter use and appropriateness in U.S. emergency departments, 1995-2010. Acad Emerg Med. 2014 Mar;21(3):292-300. doi: 10.1111/acem.12334











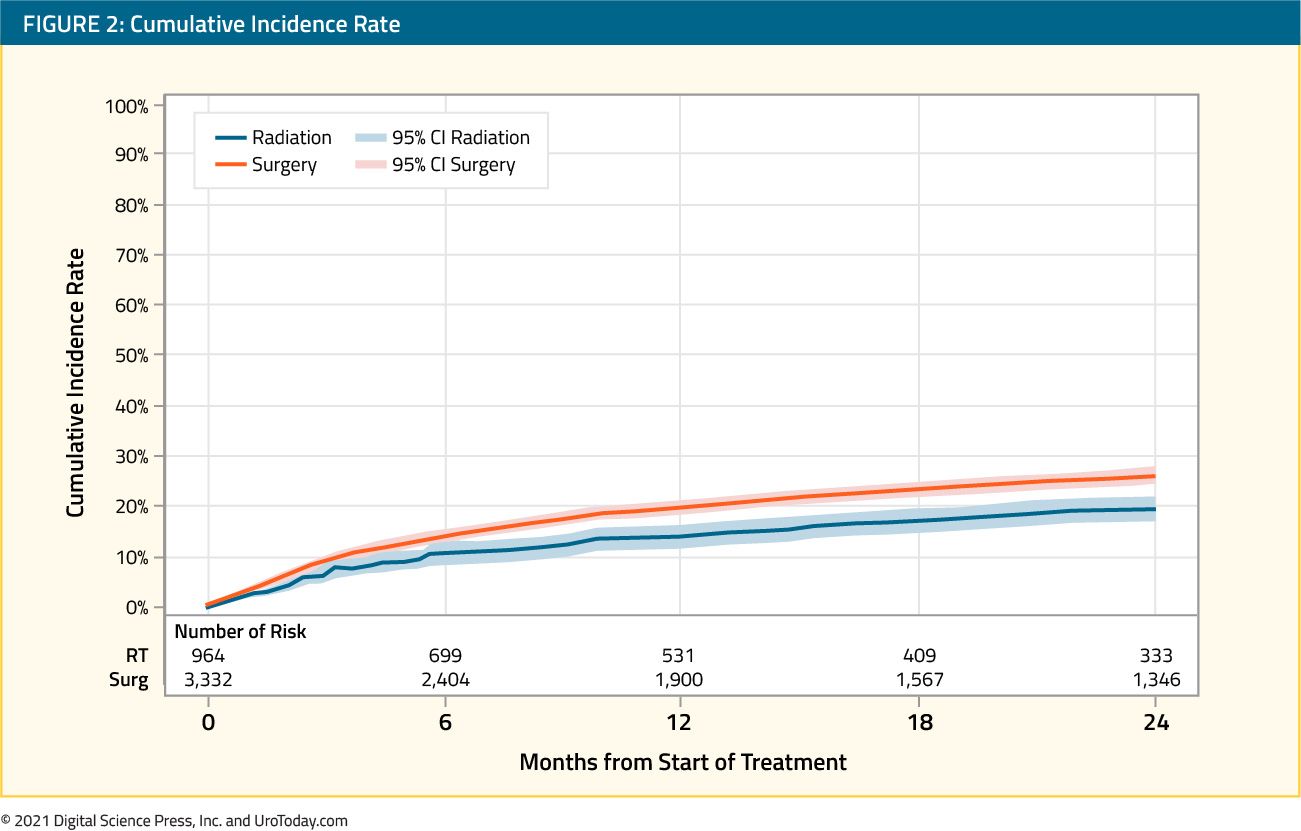


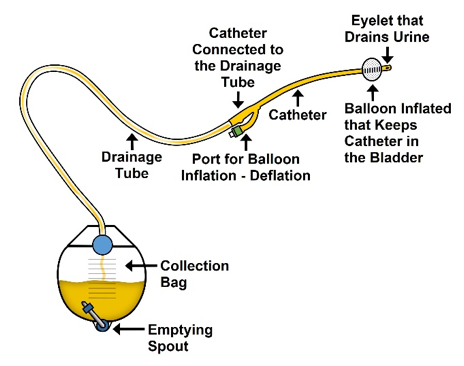




 water for inflation per manufacturer’s instructions. Larger balloons (30 cc – 60 cc) are generally used to facilitate drainage or provide hemostasis when necessary, especially in the postoperative period. The balloon of the catheter usually sits at the base of the bladder, obstructing the internal urethral orifice.
water for inflation per manufacturer’s instructions. Larger balloons (30 cc – 60 cc) are generally used to facilitate drainage or provide hemostasis when necessary, especially in the postoperative period. The balloon of the catheter usually sits at the base of the bladder, obstructing the internal urethral orifice.  infections and irritation.
infections and irritation. surgery.
surgery. drainage bag, and a spare leg strap or a device to secure the catheter tubing to the leg. Drainage bags that cannot be worn and concealed are commonly referred to as “nighttime or overnight bags,” or “large capacity bags,” or “bedside bags”. Drainage bags that can be worn and concealed are commonly referred to as “leg bags” or abdominal bags, commonly referred to as “belly bags.” Leg bags generally hold 300- 900 cc whereas an overnight bag can hold up to 2000cc. It is recommended that reusable drainage bag be replaced every 30 days.
drainage bag, and a spare leg strap or a device to secure the catheter tubing to the leg. Drainage bags that cannot be worn and concealed are commonly referred to as “nighttime or overnight bags,” or “large capacity bags,” or “bedside bags”. Drainage bags that can be worn and concealed are commonly referred to as “leg bags” or abdominal bags, commonly referred to as “belly bags.” Leg bags generally hold 300- 900 cc whereas an overnight bag can hold up to 2000cc. It is recommended that reusable drainage bag be replaced every 30 days.