Library Resources
The Treatment Landscape of Metastatic Urothelial Carcinoma: First-Line Systemic Therapy in Cisplatin Eligible Patients
Introduction
Metastatic urothelial carcinoma is associated with a poor prognosis, with a median overall survival of less than two years. To date, combination platinum-based chemotherapy remains the standard of care first line treatment for these patients who are suitable for chemotherapy. This Center of Excellence article will assess criteria for determining cisplatin chemotherapy eligibility, review the landmark trials that established cisplatin-based chemotherapy as the standard of care for first-line treatment, and review recent data for avelumab maintenance therapy among patients that did not progress on first-line chemotherapy.
- Written by: Zachary Klaassen, MD, MSc Associate Professor of Urology Urologic Oncologist Medical College of Georgia, Georgia Cancer Center Augusta, GA and Rashid Sayyid, MD, MSc Urologic Oncology Fellow University of Toronto Toronto, Ontario, Canada
- References:
- Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29: 2432-2438.
- Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol. 1985;133: 403-407.
- Sternberg CN, Yagoda A, Scher HI, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer. 1989;64: 2448-2458.
- Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17: 3173-3181.
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18: 3068-3077.
- Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. 2001;19: 2638-2646.
- Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42: 50-54.
- Lee YS, Ha MS, Tae JH, et al. Gemcitabine-cisplatin versus MVAC chemotherapy for urothelial carcinoma: a nationwide cohort study. Sci Rep. 2023;13: 3682.
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383: 1218-1230.
- Powles T, Park SH, Caserta C, et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After >/=2 Years of Follow-Up. J Clin Oncol. 2023;41: 3486-3492.
- Powles T, Sridhar SS, Loriot Y, et al. Avelumab maintenance in advanced urothelial carcinoma: biomarker analysis of the phase 3 JAVELIN Bladder 100 trial. Nat Med. 2021;27: 2200-2211.
Cretostimogene Grenadenorepvec: At the CORE and Forming BONDs in High-Risk NMIBC and PIVOTing into Intermediate Risk NMIBC
Bladder cancer remains the sixth most commonly diagnosed cancer in the United States, with an estimated 82,290 incident cases in 2023.1 Because of the persistent recurrence risk of NMIBC in a highly comorbid population, there has been an FDA-led drive towards developing novel treatment options for these patients. The following article will highlight recent advances in this disease space with a specific focus on the oncolytic adenovirus agent cretostimogene grenadenorepvec, and the registration trial in intermediate risk non-muscle invasive bladder cancer (NMIBC), PIVOT-006.
- Written by: Zachary Klaassen, MD, MSc, Wellstar MCG Health Georgia Cancer Center Augusta, Georgia, USA
- References:
- American Cancer Society. Key Statistics for Bladder Cancer. https://www.cancer.org/cancer/types/bladder-cancer/about/key-statistics.html#:~:text=time%20of%20diagnosis-,How%20common%20is%20bladder%20cancer%3F,men%20and%204%2C550%20in%20women. Accessed on December 1, 2023.
- Burke JM, Lamm DL, Meng MV, et al. A first in human phase 1 study of GC0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol. 2012 Dec;188;(6):2391-2397.
- Packiam VT, Lamm DL, Barocas DA, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol. 2018 Oct;36(10):440-447.
Novel Treatment Options for BCG Naïve Non-Muscle Invasive Bladder Cancer: Immune Priming and Immune Check Point Inhibitors
Introduction
Immune checkpoint inhibitors have emerged as a guideline-recommended first line treatment option for patients with cisplatin-ineligible, metastatic urothelial carcinoma of the bladder and as second line therapy for patients with metastatic disease progressing during, or after, platinum-based combination chemotherapy.1 Pembrolizumab, a Programmed Death-1 (PD-1) inhibitor, has been recently approved by the US Food and Drug Administration for the treatment of patients with Bacillus Calmette Guerin (BCG)-resistant non-muscle invasive bladder cancer (NMIBC), based on the results of the KEYNOTE-057 trial.2,3
Given that patients with NMIBC receiving adjuvant BCG post-TURBT have estimated risks of disease recurrence and progression of 40% and 10%, respectively,4 the BCG naïve NMIBC space may provide an opportunity to move these agents up even further along the bladder cancer disease spectrum. In this Center of Excellence article, we will summarize the current state of the evidence for ongoing trials evaluating immune check point inhibitors and other immune priming interventions in combination with BCG for the treatment of BCG naïve NMIBC.
Pembrolizumab + BCG
Previous studies have demonstrated that programmed cell death ligand 1 (PD-L1) expression is significantly increased in BCG-induced bladder granulomata of patients with BCG unresponsive disease.5 As such, it has been hypothesized that the addition of a PD-1 inhibitor, such as pembrolizumab, may overcome this potential underlying mechanism of resistance.
The combination of pembrolizumab + BCG has previously been evaluated in the setting of a phase I trial for patients with BCG unresponsive NMIBC. This combination was determined to be relatively safe, and the 13 evaluable patients had a 3-month complete response rate of 69%.6
KEYNOTE-676 is a randomized, comparator-controlled trial evaluating the efficacy and safety of pembrolizumab + BCG in patients with high-risk NMIBC (T1, CIS, high grade Ta) who underwent cystoscopy/transurethral resection of bladder tumor ≤12 weeks before randomization and had not received BCG within the preceding two years. Patients will be randomly assigned 1:1:1 to receive:
- Pembrolizumab 400 mg IV every 6 weeks + BCG reduced maintenance (≤ 6 months)
- Pembrolizumab 400 mg IV every 6 weeks + BCG full maintenance (≤ 18 months)
- BCG monotherapy with BCG full maintenance
The trial schema for KEYNOTE-676 cohort B is as follows:
The primary endpoint for this trial is event-free survival, defined as the time from random assignment to the first occurrence of any of the following:
- High-grade Ta, CIS, or any T1 disease of the bladder
- High-risk disease (high-grade Ta, CIS, or ≥T1) of the urethra or upper tract
- Locally advanced/metastatic disease determined by blinded independent central review
- Death from any cause
The secondary endpoints will include complete response rate by blinded independent central review, duration of response, disease-specific survival, time to cystectomy, overall survival, and safety.7 As follows is a geographical representation of the countries currently enrolling patients in KEYNOTE-676:
Additionally, a single arm, phase II trial (NCT03504163) from the Memorial Sloan Kettering Cancer Center is evaluating the combination of BCG + pembrolizumab in patients with high-risk T1 bladder cancer, with an additional exploratory cohort of patients with upper tract disease. This study will plan to enroll 37 patients, who will receive pembrolizumab 400 mg IV at 6-week intervals (total 9 doses), with BCG (TICE strain, 50 mg) administered once weekly for 6 weeks as induction, followed by maintenance consistent with standard clinical practice. BCG will be started on week 3 after the first infusion of pembrolizumab to allow for the initial priming of T cells to further enhance the effects of BCG treatment. The primary outcome is the proportion of patients who remain free of high-grade disease recurrences at 6 months post-treatment initiation.8
Atezolizumab + BCG
BladderGATEBladderGATE (NCT04134000) is a phase Ib-II trial evaluating the safety and efficacy of atezolizumab (anti-PD-L1) + BCG in patients with high-risk NMIBC, who are either BCG-naïve or had not received BCG in the preceding two years. Patients in this trial will receive either:
- Induction BCG with 1 instillation every week + IV atezolizumab 1,200 mg every 3 weeks (Dose level 0)
- Induction BCG with ½ instillation every week + IV atezolizumab 1,200 mg every 3 weeks (Dose level -1)
Following induction, BCG will be administered at weeks 13-15, 25-27, and 49-51, with atezolizumab concurrently administered for up to 1 year. Patients were accrued to each dose level in cohorts of 10 patients until the maximum tolerated dose is achieved (dose at which < 4 out of 10 patients experience dose-limiting toxicity). The interim safety results were recently presented at ASCO 2023. This analysis included 34 patients, with no dose-limiting toxicities reported in the first 10 patients included at dose level 0. The most frequent grade 3-4 adverse events were:
- Asthenia (9%)
- Myocarditis (3%)
- Immune-mediated hepatitis (3%)
- Hyponatremia (3%)
- Encephalopathy (3%)
- Guillain-Barre syndrome (3%)
Two patients discontinued the study treatment due to immune-mediated Grade 3 hepatitis and pneumonitis, respectively.9
ALBANALBAN (AFU-GETUG 37; NCT03799835) is a phase III trial across 30 centers in France evaluating the efficacy and safety of atezolizumab given in combination with BCG versus BCG alone in patients with BCG naïve, high-risk NMIBC (T1, high-grade, and/or CIS). Eligible patients will be randomized 1:1 to:
- Arm A: BCG alone with six weeks induction followed by three weekly maintenance instillations at 3, 6, and 12 months
- Arm B: BCG + atezolizumab (1,200 mg IV every 3 weeks for up to 1 year)
The primary endpoint is recurrence-free survival in the intent-to-treat population, with secondary efficacy endpoints of overall survival, progression-free survival, complete response, disease worsening, quality of life, and safety outcomes. Study enrollment began in December 2018 with a target of 614 patients.10
Durvalumab + BCG
POTOMAC (NCT03528694) is an open label, multicenter, randomized trial evaluating the combination of durvalumab (anti-PD-L1) and BCG in BCG-naïve patients with high-risk NMIBC (any high-grade disease, T1, CIS, LG Ta if >3 cm, recurrent, and multifocal). This trial will randomize 1,018 patients to:
- BCG induction + maintenance for 24 months
- BCG induction + maintenance + durvalumab (1,500 mg every 4 weeks for 13 cycles)
- BCG induction only (no maintenance) + durvalumab
The study design is as follows:
The primary study endpoint is disease-free survival, with secondary endpoints including the proportion of patients alive and disease-free at 24 months, 5-year overall survival, pharmacokinetics, immunogenicity, safety, tolerability, and health-related quality of life.11
Sasanlimab + BCG
Sasanlimab is an anti-PD-1 monoclonal antibody that has demonstrated an acceptable safety profile and promising clinical activity in patients with locally advanced or metastatic urothelial carcinoma, within the context of a phase 1 trial.12 The phase 3 CREST study (NCT04165317) Cohort A will evaluate subcutaneous injection sasanlimab in patients with BCG naive NMIBC. Patients in this cohort will be randomized to one of three arms:
- Arm A: Sasanlimab + BCG induction + maintenance
- Arm B: Sasanlimab + BCG induction only
- Arm C: BCG induction + maintenance
This trial will assess for between-arm differences in event-free, disease-specific, and overall survivals, complete response rate, adverse events/safety profile, and health-related quality of life.13 Of note, On August 31, 2022, the Sponsor announced the discontinuation of enrollment to Part B (Cohort B), which enrolled participants with BCG unresponsive NMIBC. The decision to discontinue enrollment to Part B (Cohort B) was not made for safety reasons.14
Immune Priming: Intradermal BCG
The PRIME trial (SWOG S1612) is evaluating whether intradermal BCG inoculation may potentiate the immune effects of subsequent intravesical BCG instillations. This hypothesis was recently tested in a single arm trial that demonstrated that percutaneous BCG administered 21 days prior to intravesical instillation in patients with high-risk NMIBC boosted BCG-specific immunity at 3 months and increased the activation status of in vitro expanded circulating NK and γδ T cells and their cytotoxicity against bladder cancer cells.15
In the PRIME trial, the Tokyo BCG strain is being used for both intradermal inoculation and intravesical instillation in a three-arm design with TICE strain BCG also used as a standard of care comparator. This study was designed to test:
- The comparitive superiority between intravesical Tokyo strain BCG when combined with intradermal inoculation as compared to intravesical alone (arms 2 and 3)
- The non-inferiority of intravesical Toyko strain alone to intravesical TICE strain (arms 1 and 2).
- TICE strain is currently the only strain that is FDA approved and in production in the USA (Armond-Frappier and Connaught are also FDA approved, but not currently in production). As such, if the Tokyo strain is found to be non-inferior to TICE, this may facilitate its subsequent FDA approval and increased the availability of additional BCG strains during the current shortage
The study design is as follows:
This trial has now fully accrued and is awaiting readout in the nearby future.
Conclusions
Numerous ongoing trials are evaluating the combination of BCG and an immune checkpoint inhibitor for patients with BCG naïve NMIBC. By inhibiting the PD-1/PD-L1 axis, it is hypothesized that these agents may help overcome underlying mechanisms of resistance inherent to BCG-resistant strains, and thus improve on the historic recurrence and progression rates observed with BCG treatment alone. Many of these trials have completed accrual, and we await the results of these combinatory trials in the upcoming years.
- Written by: Rashid K. Sayyid, MD, MSc, University of Toronto, Toronto, ON & Zachary Klaassen, MD, MSc, Medical College of Georgia, Augusta, Georgia, USA
- References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer. https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer. Accessed on Aug 6, 2023.
- FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer. Accessed on Aug 6, 2023.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22(7):919-30.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7–H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499-505
- Alanee S, Sana S, El-Zawahry A, et al. Phase I trial of intravesical Bacillus Calmette-Guérin combined with intravenous pembrolizumab in recurrent or persistent high-grade non-muscle-invasive bladder cancer after previous Bacillus Calmette-Guérin treatment. World J Urol. 2021;39(10):3807-13.
- Kamat AM, Shariat S, Steinberg GD, et al. Randomized comparator-controlled study evaluating efficacy and safety of pembrolizumab plus Bacillus Calmette-Guérin (BCG) in patients with high-risk nonmuscle-invasive bladder cancer (HR NMIBC): KEYNOTE-676 cohort B. J Clin Oncol. 2022;40(Suppl 6): TPS597
- ClinicalTrials.gov - Pembrolizumab (MK-3475) and Bacillus Calmette-Guérin (BCG) as First-Line Treatment for High-Risk T1 Non-Muscle-Invasive Bladder Cancer (NMIBC) and High-Grade Non-Muscle-Invasive Upper Tract Urothelial Carcinoma (NMI-UTUC)].
- Castellano D, de Velasco G, Carretero-Gonzalez A, et al. Atezolizumab + intravesical BCG (bacillus Calmette-Guerin) upfront combination in high risk non–muscle- invasive bladder cancer (NMIBC) patients: Safety interim report of BladderGATE phase I-II study. J Clin Oncol. 2023;41(Supp 16):e16590.
- Roupret M, Neuzillet Y, Bertaut A, et al. ALBAN: An open label, randomized, phase III trial, evaluating efficacy of atezolizumab in addition to one year BCG (Bacillus Calmette-Guerin) bladder instillation in BCG-naive patients with high-risk nonmuscle invasive bladder cancer (AFU-GETUG 37). J Clin Oncol. 2019;37(Suppl 15):TPS4589.
- De Santis M, Abdrashitov R, Hegele A, et al. A phase III, randomized, open-label, multicenter, global study of durvalumab and bacillus calmette-guérin (BCG) versus BCG alone in high-risk, BCG-naïve non-muscle-invasive bladder cancer (NMIBC) patients (POTOMAC). J Clin Oncol. 2019;37(Suppl 7):TPS500.
- Shore ND, Powles T, Bedke J, et al. A phase 3 study of the subcutaneous programmed cell death protein 1 inhibitor sasanlimab as single agent for patients with bacillus Calmette-Guérin, unresponsiv,e high-risk, non-muscle invasive bladder cancer: CREST Study Cohort B. J Clin Oncol. 2022;40(Suppl 16):40.
- ClinicalTrials.gov. A Study of Sasanlimab in People With Non-muscle Invasive Bladder Cancer (CREST). https://classic.clinicaltrials.gov/ct2/show/NCT04165317. Access on Aug 6, 2023.
-
Clinicaltrials.gov. Available at: https://www.clinicaltrials.gov/study/NCT04165317?intr=sasanlimab&rank=8 (Accessed: 26 April 2024)
Novel Targets and Treatment Developments in Metastatic Hormone Sensitive Prostate Cancer
Introduction
The last decade has seen a seismic shift in the treatment landscape of metastatic hormone sensitive prostate cancer (mHSPC). This includes several guideline and FDA approved doublet therapy options, triplet therapy options, and treatment with radiotherapy to the primary tumor:- Written by: Zachary Klaassen, MD, MSc Associate Professor of Urology Urologic Oncologist Medical College of Georgia, Georgia Cancer Center Augusta, GA and Rashid Sayyid, MD, MSc Urologic Oncology Fellow University of Toronto Toronto, Ontario, Canada
- References:
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2019;381(1):13-24.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39(20):2294-2303.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121-131.
- Sweeney CJ, Martin AJ, Stockler MR, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(4):323-334.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974-2986.
- Armstrong AJ, Iguchi T, Azad AA, et al. The Efficacy of Enzalutamide plus Androgen Deprivation Therapy in Oligometastatic Hormone-sensitive Prostate Cancer: A Post Hoc Analysis of ARCHES. Eur Urol. 2023;84(2):229-241.
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352-360.
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686-700.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022;386(12):1132-1142.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Med. 2022;19(6):e1003998.
- Armenia J, Wankowicz SAM, Liu D, et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet. 2018;50(5):645-651.
- Chung JH, Dewal N, Sokol E, et al. Prospective Comprehensive Genomic Profiling of Primary and Metastatic Prostate Tumors. JCO Precis Oncol. 2019;3.
- Kumar A, White TA, MacKenzie AP, et al. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci U S A. 2011;108(41):17087-17092.
- Hamid AA, Sayegh N, Tombal B, et al. Metastatic Hormone-Sensitive Prostate Cancer: Toward an Era of Adaptive and Personalized Treatment. Am Soc Clin Oncol Educ Book. 2023;43:e390166.
- Hamid AA, Gray KP, Shaw G, et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur Urol. 2019;76(1):89-97.
- Velez MG, Kosiorek HE, Egan JB, et al. Differential impact of tumor suppressor gene (TP53, PTEN, RB1) alterations and treatment outcomes in metastatic, hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 2022;25(3):479-483.
- Hamid AA, Huang HC, Wang V, et al. Transcriptional profiling of primary prostate tumor in metastatic hormone-sensitive prostate cancer and association with clinical outcomes: correlative analysis of the E3805 CHAARTED trial. Ann Oncol. 2021;32(9):1157-1166.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020.
- Dhiantravan N, Emmett L, Joshua AM, et al. UpFrontPSMA: a randomized phase 2 study of sequential (177) Lu-PSMA-617 and docetaxel vs docetaxel in metastatic hormone-naive prostate cancer (clinical trial protocol). BJU Int. 2021;128(3):331-342.
Novel Treatment Options for BCG Naïve Non-Muscle Invasive Bladder Cancer: Intravesical Chemotherapy
Introduction
Bacillus Calmette Guerin (BCG) is currently guideline-recommended in the adjuvant setting for patients with intermediate or high-risk non-muscle invasive bladder cancer (NMIBC).1 This is based on the results of numerous randomized clinical trials and meta-analyses demonstrating its ability to reduce the rates of disease recurrence and progression, compared to transurethral resection of bladder tumor (TURBT) alone or other adjuvant therapies.2-5
- Written by: Rashid K. Sayyid, MD, MSc University of Toronto Toronto, ON & Zachary Klaassen, MD, MSc Medical College of Georgia Augusta, Georgia, USA
- References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer. https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer. Accessed on Aug 5, 2023.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Schmidt S, Kunath F, Coles B, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Review. 2020;1(1):CD011935.
- Malmstrom PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56(2):247-56.
- Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169(1):90-5.
- Oresta B, et al. Sci Transl Med. Jan 6;13(575):eaba6110 Clinical trial information: EudraCT 2021-003751-42_studio ICH-013 (MMC).
- Di Stasi SM, Giannantoni A, Giurioli A, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: a randomised controlled trial. Lancet Oncol. 2006;7:43-51.
- Solsona E, Madero R, Chantada V, et al. Sequential combination of mitomycin C plus bacillus Calmette-Guérin (BCG) is more effective but more toxic than BCG alone in patients with non-muscle-invasive bladder cancer in intermediate- and high-risk patients: final outcome of CUETO 93009, a randomized prospective trial. Eur Urol. 2015;67(3):508-16.
- Kaasinen E, Wijkstrom H, Rintala E, et al. Seventeen-year follow-up of the prospective randomized Nordic CIS study: BCG monotherapy versus alternating therapy with mitomycin C and BCG in patients with carcinoma in situ of the urinary bladder. Scand J Urol. 2016;50(5):360-8.
- De Nunzio C, Leonardo C, Carbone A, et al. MP63-12 THE EFFECTS OF SEQUENTIAL MITOMYCIN AND BACILLUS CALMETTE-GUÉRIN TREATMENT VERSUS BACILLUS CALMETTE-GUÉRIN MONOTHERAPY IN PATIENTS WITH HIGH-RISK NON-MUSCLE INVASIVE BLADDER CANCER: MITO-BCG (EUDRACT-2017-004540-37). J Urol. 2023;209(Suppl 4):e876.
- Jagannath C, Lindsey DR, Dhandayuthapani S, et al.. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–76.
- Ji N, Mukherjee N, Reyes RM, et al. Rapamycin enhances BCG-specific γδ T cells during intravesical BCG therapy for non-muscle invasive bladder cancer: a randomized, double-blind study. J Immunother Cancer. 2021;9(3):e001941.
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-Institution Evaluation of Sequential Gemcitabine and Docetaxel as Rescue Therapy for Nonmuscle Invasive Bladder Cancer. J Urol. 2020;203(5):902-9.
- McElree IM, Steinberg RL, Mott SL, et al. Comparison of Sequential Intravesical Gemcitabine and Docetaxel vs Bacillus Calmette-Guérin for the Treatment of Patients With High-Risk Non–Muscle-Invasive Bladder Cancer. JAMA Netw Open. 2023;6(2):e230849.
- Guerrero-Ramos F, Gonzalez-Padilla DA, Gonzalez-Diaz A, et al. Recirculating hyperthermic intravesical chemotherapy with mitomycin C (HIVEC) versus BCG in high-risk non-muscle-invasive bladder cancer: results of the HIVEC-HR randomized clinical trial. World J Urol. 2022;40(4):999-1004.
Novel Treatment Targets in the Metastatic Castrate-Resistant Prostate Cancer Disease Space
Introduction
Since the United States Food and Drug Administration (FDA) approval of mitoxantrone in 19961 and docetaxel in 20042 for the treatment of patients with metastatic castrate-resistant prostate cancer, we have witnessed the approval of numerous additional agents/combinations in this disease space:
- Written by: Rashid K. Sayyid, MD MSc University of Toronto Toronto, ON & Zachary Klaassen, MD MSc Georgia Cancer Center Wellstar MCG Health Augusta, Georgia
- References:
- Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996:14(6):1756-1764.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376(9747):1147-1154.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433.
- Center for Drug Evaluation and Research. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. U.S. Food and Drug Administration.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol. 2020;38(32):3763-3772.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091-2102.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385(12):1091-1103.
- Clarke N, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence. 2022.EVIDoa2200043.
- Agarwal N, Azad A, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. The Lancet. 2023;402(10398):291-303.
- George DJ, Sartor O, Miller K, et al. Treatment Patterns and Outcomes in Patients With Metastatic Castration-resistant Prostate Cancer in a Real-world Clinical Practice Setting in the United States. Clin Genitourin Cancer. 2020;18(4):284-94.
- Koivisto P, Kononen J, Palmberg C, et al. Androgen Receptor Gene Amplification: A Possible Molecular Mechanism for Androgen Deprivation Therapy Failure in Prostate Cancer. Cancer Res. 1997;57(2):314-9.
- Henzler C, Li Y, Yang R, et al. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. Nat Commun. 2016;7:13668.
- Pachynski RK, Iannotti N, Laccetti AL, et al. Oral EPI-7386 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2023;41(Suppl 6):177.
- Laccetti AL, Chatta GS, Iannotti N, et al. Phase 1/2 study of EPI-7386 in combination with enzalutamide (enz) compared with enz alone in subjects with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(Suppl 6):179.
- Desai K, Serritella AV, Stadler WM, et al. Phase I trial of enzalutamide (Enz) plus the glucocorticoid receptor antagonist relacorilant (Rela) for patients with metastatic castration resistant prostate cancer. J Clin Oncol. 2023;41(Suppl 6):5062.
- Fizazi K, Cook N, Barthelemy P, et al. Phase 1 results of the ODM-208 first-in-human phase 1-2 trial in patients with metastatic castration-resistant prostate cancer (CYPIDES). J Clin Oncol. 2022;40(Suppl 6):18.
- Smith MR, Agarwal N, Todenhofer T, et al. CYCLONE 2: A phase 2/3, randomized, placebo-controlled study of abiraterone acetate plus prednisone with or without abemaciclib in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2022;40(Suppl 6):198.
- Dorff TB, Blanchard S, Martirosyan H, et al. Final results from phase I study of PSCA-targeted chimeric antigen receptor (CAR) T cells in patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(Suppl 6):5019.
- Sandhu S, Joshua AM, Emmett L, et al. LuPARP: Phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(Suppl 6):5005.
- Kostos LK, Buteau JP, Kong G, et al. LuCAB: A phase I/II trial evaluating cabazitaxel in combination with [177Lu]Lu-PSMA-617 in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(Suppl 6):TPS278.
- Teiluf K, Seidl C, Blechert B, et al. α-Radioimmunotherapy with 213Bi-anti-CD38 immunoconjugates is effective in a mouse model of human multiple myeloma. Oncotarget. 2015;6:4692-4703.
- Ma J, Li L, Liao T, Fong W, Zhang C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2022;12:796657.
- Nauseef JT, Sun MP, Thomas C, et al. A phase I/II dose-escalation study of fractionated 225Ac-J591 for progressive metastatic castration-resistant prostate cancer (mCRPC) in patients with prior treatment with 177Lu-PSMA. J Clin Oncol. 2023;41(Supp 6):TPS288.
The Path Forward for Telehealth in Medicare
The Current State of Treatment Implementation for mCRPC in North America
Introduction
There have been significant advances in the metastatic castrate-resistant prostate cancer (mCRPC) treatment landscape with the emergence and approval of numerous agents in this disease space.- Written by: Rashid Sayyid, MD MSc University of Toronto Toronto, ON & Zachary Klaassen, MD MSc Georgia Cancer Center Wellstar MCG Health Augusta, GA
- References:
- Freedland SJ, Davis M, Epstein AJ, et al. Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population. Prostate Cancer Prostatic Dis. 2023.
- FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate. Accessed on October 29, 2023.
- FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer. Accessed on October 29, 2023.
- FDA D.I.S.C.O. Burst Edition: FDA approval of Lynparza (olaparib), with abiraterone and prednisone, for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-lynparza-olaparib-abiraterone-and-prednisone-brca-mutated#:~:text=On%20May%2031%2C%202023%2C%20the,FDA%2Dapproved%20companion%20diagnostic%20test.. Accessed on October 29, 2023.
- FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate. Accessed on October 29, 2023.
- FDA approves Pluvicto for metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer. Accessed on October 29, 2023.
- Swami U, Aggarwal H, Zhou M, et al. Treatment Patterns, Clinical Outcomes, Health Care Resource Utilization and Costs in Older Patients With Metastatic Castration-Resistant Prostate Cancer in the United States: An Analysis of SEER-Medicare Data. Clin Genitourin Cancer. 2023;21(5):517-529.
- Shayegan B, Wallis CJD, Malone S, et al. Real-world use of systemic therapies in men with metastatic castration resistant prostate cancer (mCRPC) in Canada. Urol Oncol. 2022;40(5):192.e1-192.e9.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-1512.
- Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20(12):1730-1739.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091-2102.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N Engl J Med. 2023;388:719-732.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376(9747):1147-1154.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med. 2019;381(26):2506-2518.
Medicare Advantage: the Who, What, Where, Why… and What’s Next?
The Current State of Treatment Implementation for Metastatic Hormone Sensitive Prostate Cancer in North America
Introduction
Since 1941, the backbone of treatment for advanced prostate cancer has been androgen deprivation therapy (ADT). However, treatment advancement remained relatively stagnant until the last decade, when we saw the emergence of several doublet and triplet therapy options, using ADT as the backbone of treatment, leading to an overall survival (OS) advantage versus ADT alone.Figure 1: The current landscape of FDA combination approvals in Metastatic Hormone Sensitive Prostate Cancer (mHSPC)1-12 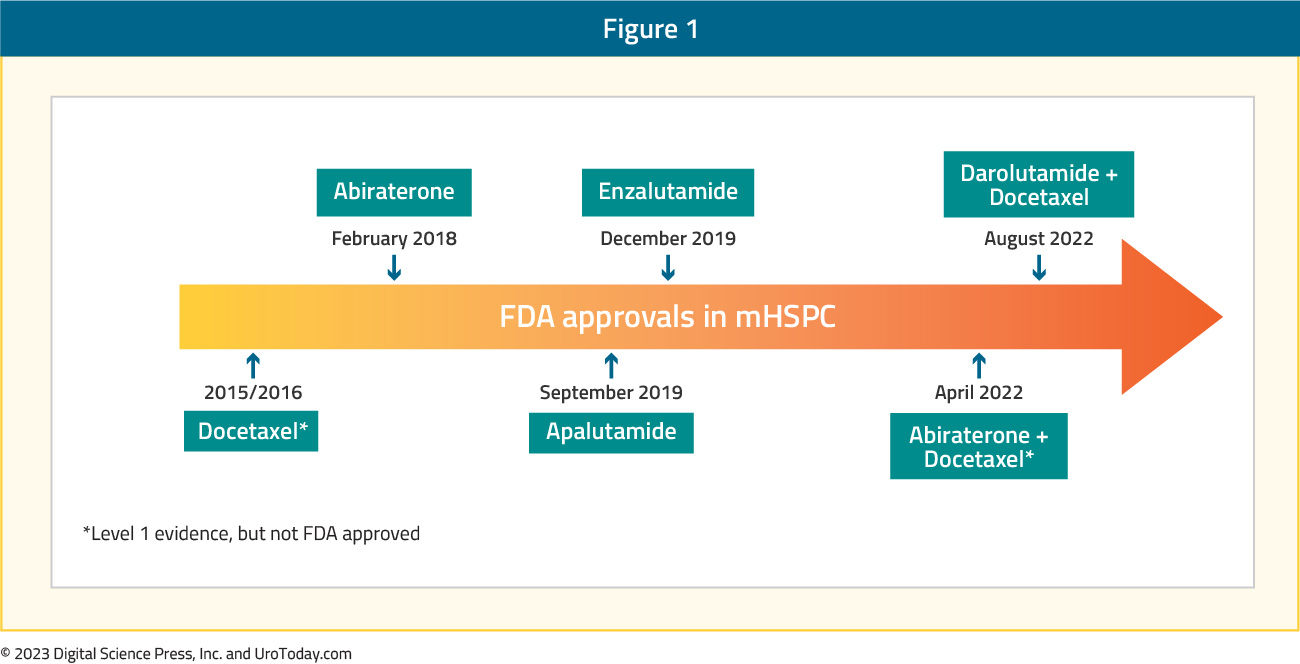
Thus, this has changed the standard of care for treatment intensification for these men. This article will focus a discussion on the implementation of treatment for Metastatic Hormone Sensitive Prostate Cancer (mHSPC) in North America, specifically highlighting the landscape and challenges of treatment intensification, and the importance of disease volume and timing of metastasis for selecting the optimal treatment in the mHSPC disease space.
The Enigma of (Lack of) Treatment Intensification
Despite the approval and availability of multiple mHSPC treatment intensification strategies, there remains a clear underutilization of these combination strategies in real-world practice. The following discussion will highlight several of the key studies looking at contemporary treatment intensification across several North American jurisdictions.
Ryan et al. reported treatment utilization trends of mHSPC patients between January 2014 and July 2019 from two large U.S databases: (i) Optum’s de-identified Clinformatics Data Marta Database (COM/MA), which includes claims from commercial and Medicare Advantage plans for 13 million people across the United States and (ii) Centers for Medicare & Medicaid Services-sourced Medicare Fee-for-Service (FFS) Research Identifiable Files Sample.13 A total of 19,841 mHSPC patients (6,517 COM/MA and 13,324 Medicare-FFS) were identified with a median follow up of 9.6 – 10.5 months. Notably, 38% of COM/MA and 48% of Medicare-FFS patients remained untreated or deferred treatment during the study period, whereas 45% and 46%, respectively, were treated with first line ADT monotherapy only. Abiraterone acetate or docetaxel was used as first line therapy in 13% (COM/MA) and 2%
(Medicare-FFS) of patients. Approximately 43% and 38% of patients, respectively, only received ADT monotherapy (with or without a first-generation androgen signaling inhibitors) during the entire mHSPC period despite the availability of other more potent therapies. It is important to note that apalutamide and enzalutamide were added to evidence-based guidelines as mHSPC treatments after the end of the study period (July 31, 2019) and were not considered as mHSPC therapy in the current study. When stratified by year of index date, in the COM/MA database, treatment with first line ADT monotherapy decreased numerically from 48% to 43% among patients diagnosed with mHSPC in 2015 – 2017 versus 2018 – 2019. While the overall use of abiraterone or docetaxel remained similar in the two periods (12% - 14%), the relative use of abiraterone acetate increased among patients diagnosed in 2018 - 2019 (10%) versus 2015 - 2017 (5%), whereas the use of docetaxel decreased in 2018 - 2019 (4%) compared with 2015 – 2017 (7%).
Figure 2: First line therapy in patients with mHSPC by year of index data in the Clinformatics Data Marta Database13 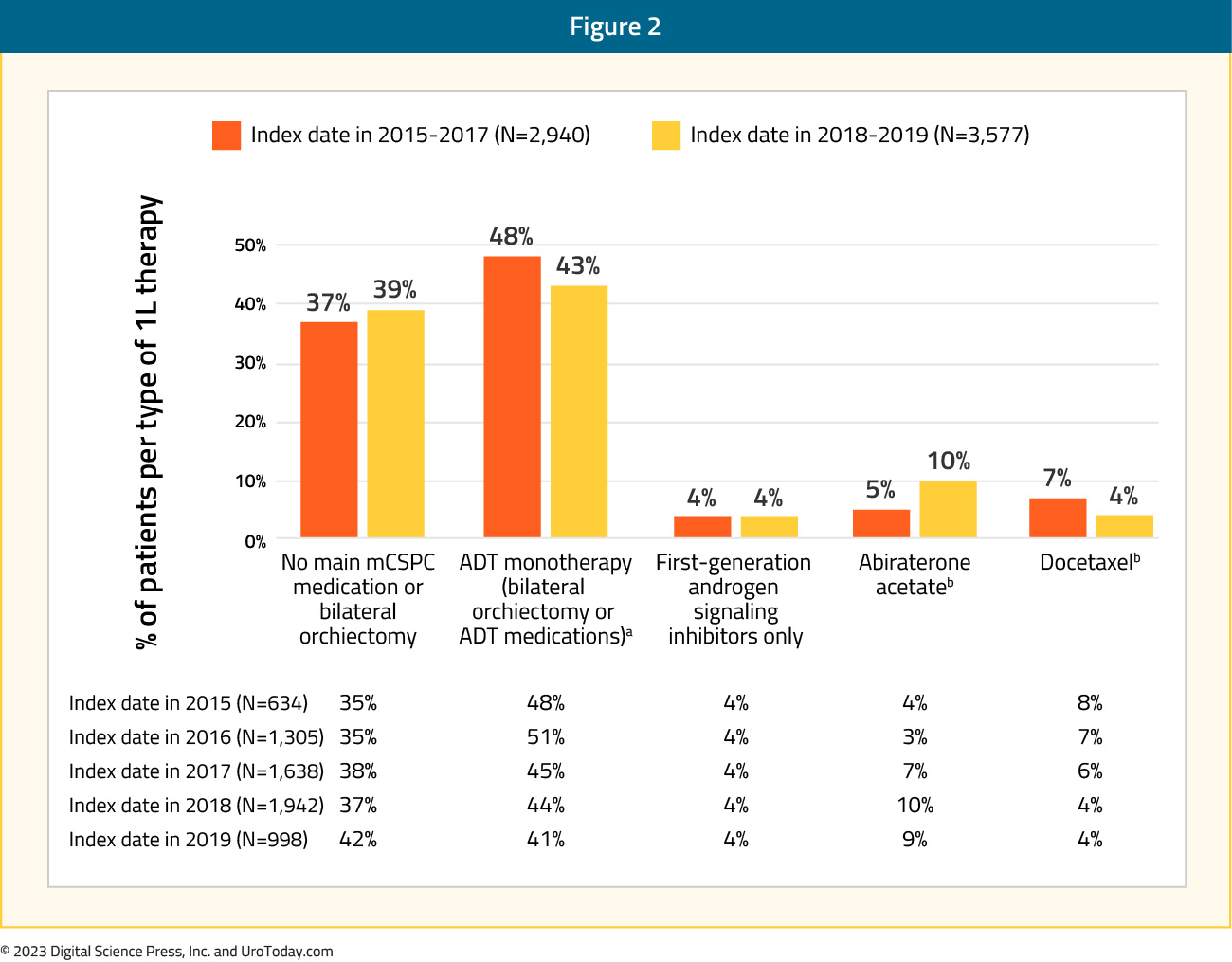
Analysis from the Veterans Health Administration (VHA) database was reported by Freedland et al.14 for 1,395 mHSPC patients treated between April 2013 and March 2018. Across the five-year study period, 63% of patients received ADT alone as first line treatment while ADT + non-steroidal anti-androgen was used in 24%, ADT + docetaxel in 8%, and ADT + abiraterone for the remaining 5%. Treatment trends over time did demonstrate an overall decrease in ADT only (66% to 60%) or ADT + non-steroidal anti-androgen (31% to 17%) utilization between 2014 and 2017-2018, with a corresponding increase in utilization of ADT+ docetaxel (3% to 9%) and ADT + abiraterone (1% to 15%). Nonetheless, despite increased adoption of treatment intensification in VHA mHSPC patients, there remains a clear underutilization of appropriate treatment intensification.
Figure 3: Treatment trends over time among patients with mHSPC in the Veterans Health Administration14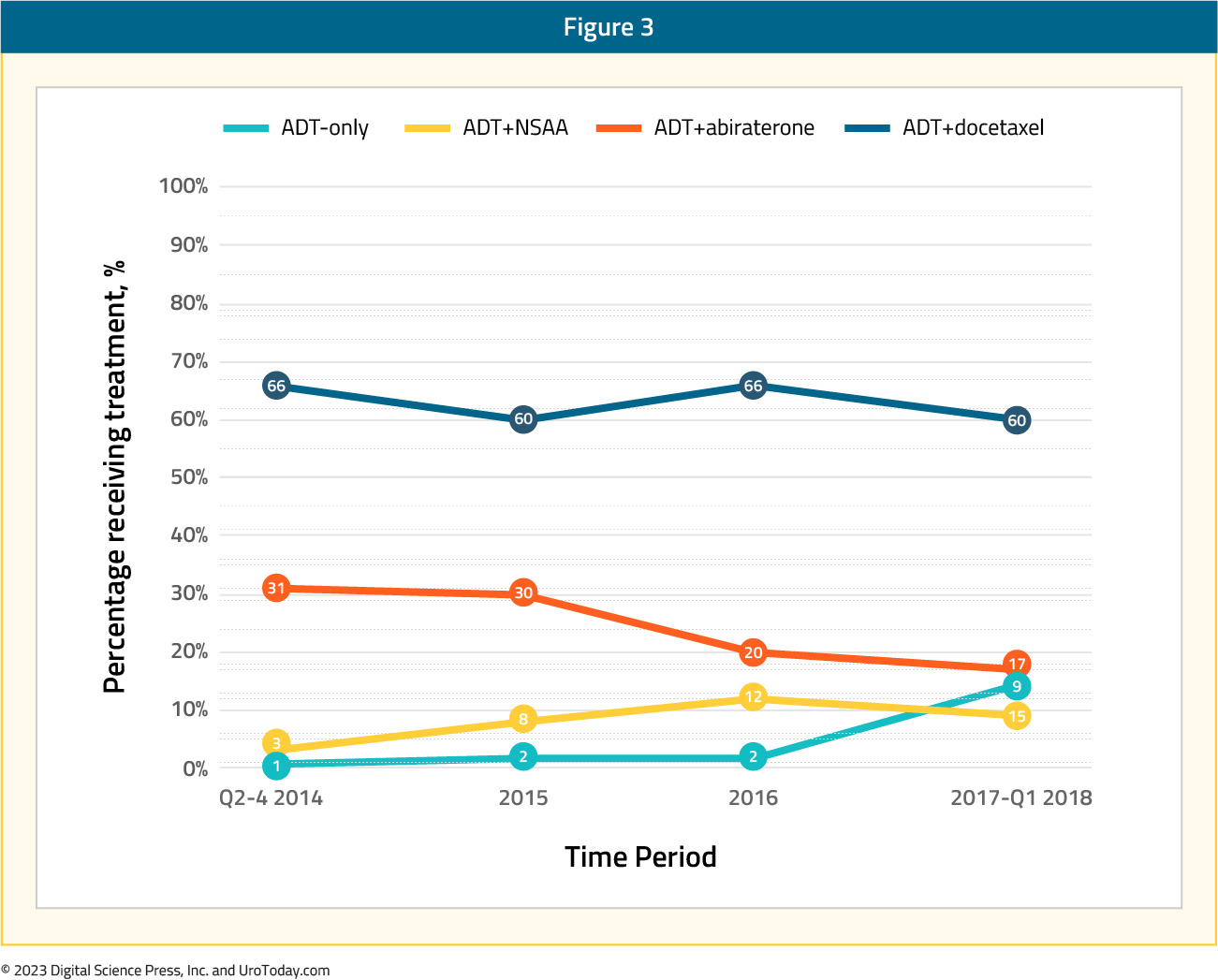
A comparison of patients in the four treatment groups demonstrated that patients receiving ADT and docetaxel, compared to the ADT only cohort, were younger (65.8 versus 73.4 years) and had fewer comorbidities (National Cancer Institute comorbidity score 1.1 versus 1.5), but had greater disease burden in terms of higher PSA (338.1 ng/ml versus 256.4 ng/ml) and overall metastatic burden. ADT + abiraterone patients were older (75.3 versus 73.4 years), generally had fewer cardiovascular comorbidities and lower PSA (238.3 versus 256.5 ng/ml) but had increased metastasis (other sites including bone: 80% versus 73%).
A combined analysis from the VHA and Medicare database was presented at ESMO 2022. The authors identified 33,641 and 5,561 men in the Medicare and VA cohorts, respectively. Similar to the prior report by Freedland et al., 14 the authors demonstrated that the proportion of patients receiving treatment intensification with novel hormonal therapy or docetaxel increased over time, although by 2018/2019, still less than one third of patients with mHSPC received first line ADT plus docetaxel or ADT plus a novel hormonal agent.
Figure 4: Utilization of first line treatment intensification over time for mHSPC patients in the VHA and Medicare databases
While there were changes in treatment approaches between 2010 and 2014, the authors did not find any changes in overall survival in 2012–2014 compared with 2010–2011, though there was improvement in overall survival by 12% and 15% in the Medicare and VA cohorts, respectively, in 2015–2018/2019 versus 2010–2011, after adjusting for baseline characteristics, suggesting that diffusion of these intensified treatment approaches provides a population-level survival benefit.
Figure 5: Overall survival for mHSPC patients in the VHA and Medicare databases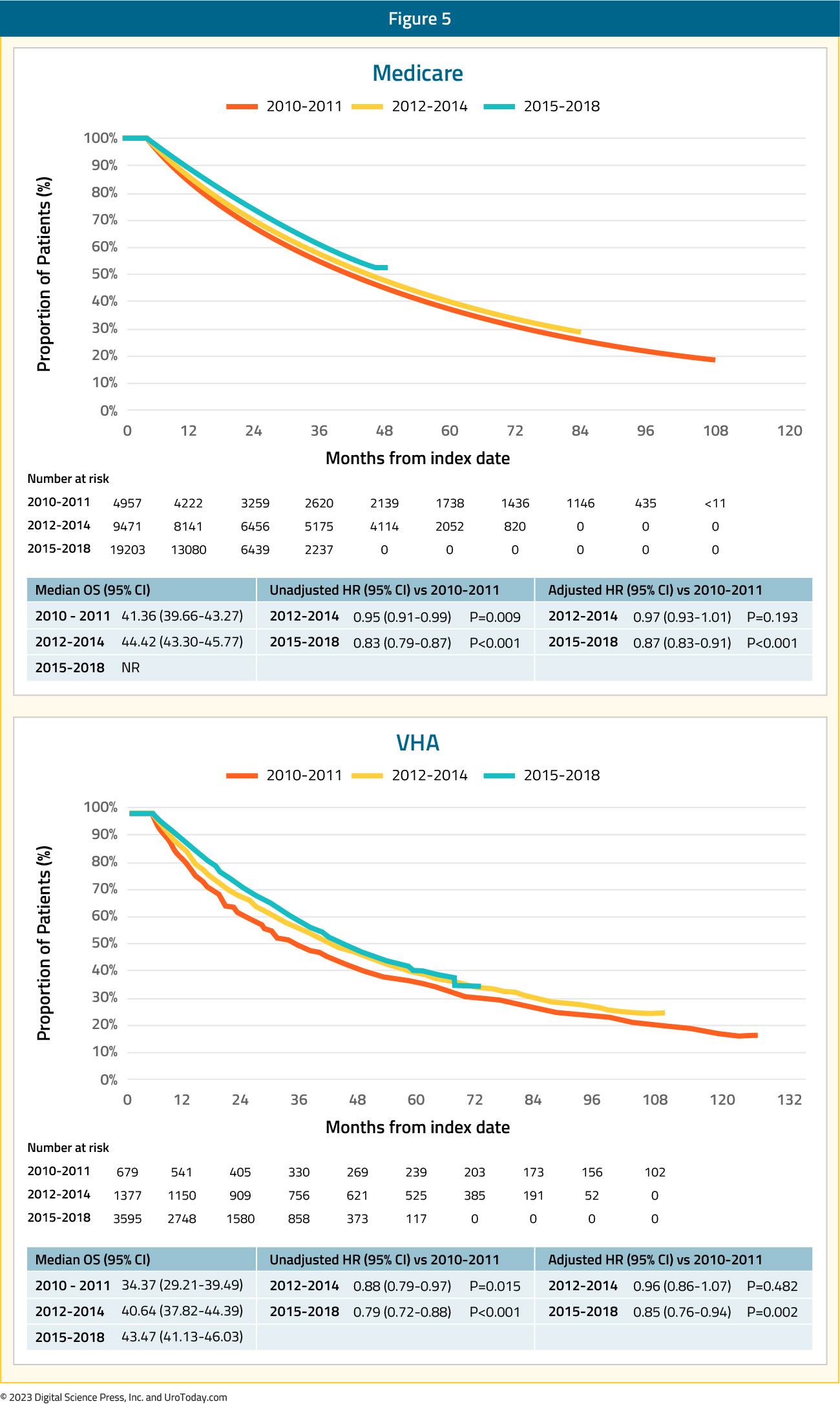
A Medicare analysis of treatment intensification trends among racial minorities was presented by Freedland et al. at ASCO 2021. Compared to White, non-Hispanic men, Black men were less likely to receive treatment intensification (ADT + docetaxel or novel hormonal therapy) during 2010-2014 (2.6% versus 3.2%), 2015-2016 (8.7% versus 10.4%), and 2017 (14.2% versus 15.1%).
Using provincial data from Ontario, Canada, Wallis et al.15 identified 3,556 patients diagnosed with de novo mHSPC between 2014 and 2019. Of note, 78.6% of patients received ADT alone (with or without an anti-androgen), 11.2% received treatment intensification with docetaxel, 1.5% received abiraterone acetate and prednisone, with the remaining 8.7% receiving a “non-ADT” regimen. Patients receiving docetaxel were comparatively younger (mean age 72.6 years) and healthier (mean Charlson Comorbidity Index score of 0.15). The median PSA at diagnosis was lower among patients who received conventional ADT (88 ng/mL) compared with ADT intensification regimens (121 ng/mL and 152 ng/mL for the abiraterone and docetaxel cohorts, respectively). A time-stratified analysis representing the uptake of ADT intensification regimens before and after the pivotal 2017 LATITUDE trial,8 demonstrated that abiraterone acetate plus prednisone prescriptions increased from 0.5% to 3% in the pre- versus post-LATITUDE period, respectively, whereas docetaxel treatment dropped from 12% to 10%. As was reported by Ryan et al.13, these data suggest that the pivotal data from LATITUDE and STAMPEDE resulted in a substitution of intensification approach (from docetaxel to abiraterone) rather than a broadening of the patient population receiving treatment intensification.
Given the underwhelming utilization of treatment intensification for mHSPC patients in the real-world setting, barriers to improved adoption need to be further understood. At ASCO 2022, Freedland et al. provided the first granular assessment as to reasons for or against treatment intensification. This study examined data from medical charts of patients initiating mHSPC treatment from July 2018 to November 2021 based on a retrospective review of multiple US academic/community practices. This was a survey of oncologists and urologists who treated these patients to provide reasons for treatment choices, including PSA goals and explicit reasons for not prescribing novel hormonal agents. This analysis included 621 patients who were treated by 65 oncologists and 42 urologists. In the first line setting, most mHSPC patients received ADT ± non-steroidal anti-androgen alone (69%), while treatment intensification rates with ADT + novel hormonal agent (26%) or ADT + chemohormonal therapy (4%) were low. Following the initial treatment course, an additional 166 patients (27%) received subsequent treatment intensification while still castration-sensitive, prior to progression to castration resistant disease.
When the physicians were queried about reasons for not using novel hormonal agents, the most frequently cited reasons were:
- “Novel hormonal therapy would need to have a better/more tolerable side effect profile/fewer adverse events than my chosen regimen” (38%)
- “I would need to have seen clinical trial evidence of survival improvements on novel hormonal therapies including a wider range of prostate cancer patients” (31%)
- “Novel hormonal therapies would need to be reimbursed by patients’ insurance” (26%)
Figure 6: Reasons given by providers for not using novel hormonal therapy
Regarding treatment goals for PSA response, physicians more frequently reported a relative reduction than an absolute PSA reduction (85% versus 51%). Oncologists considered a median PSA reduction of 50% (IQR 25-75%) adequate versus 75% (IQR 50-90%) among urologists. Urologists were more likely to utilize treatment intensification in the first line setting or subsequently in patients who were still castration-sensitive (p < 0.01). Furthermore, physicians who aimed for deeper PSA reductions of 75-100% were more likely (OR: 1.63, p = 0.034) to provide treatment intensification in the first-line setting compared with physicians with less aggressive PSA goals (0 – 49%). The authors concluded that physician survey results suggest that perceptions of tolerability and lack of efficacy and financial considerations affect novel hormonal therapy use. In practice, non-guideline driven PSA reduction goals are associated with low rates of treatment intensification, and these results clearly demonstrate the need for further medical education.
Treating Implementation: Using Disease Volume and Timing of Metastasis to Guide Treatment Selection
Patients with mHSPC at the time of diagnosis are defined as having de novo or synchronous metastatic disease. Additionally, there is a subset of men initially diagnosed with non-metastatic disease, many of whom had received prior definitive local treatment, who will have progression to a metastatic state prior to development of castration resistance; this is known as metachronous mHSPC. This distinction between synchronous (i.e. de novo) and metachronous presentations is of utmost clinical importance given the known differences in underlying genomic mutational profiles and prognoses, influencing the subsequent choice of treatment intensification.16 These two cohorts can be further subdivided based on the volume of metastatic disease at presentation: low and high volumes. The CHAARTED high-volume criteria have been widely adopted in clinical practice, with high volume patients defined as follows: presence of visceral metastases or ≥4 bone lesions with ≥1 beyond the vertebral bodies and pelvis.17
As such four distinct subgroups become clinically relevant (median OS per CHAARTED and GETUG-15 among men receiving ADT alone, i.e., the control groups in these trials):
- Synchronous and high volume: 3 years
- Synchronous and low volume: 4.5 year
- Metachronous and high volume: 4.5 years
- Metachronous and low volume: ~8 years
While early, aggressive treatment intensification with triplet regimens, with or without primary radiotherapy, may seem attractive in this cohort of patients to maximize survival outcomes, the reality is that such “maximal” treatment intensification is unnecessary in the majority of these patients. Furthermore, treatment toxicity, both from a pathophysiologic and financial standpoint, must be considered in these patients. As such, a nuanced approach to the treatment of such patients, guided by the aforementioned four presentations (synchronous high volume, synchronous low volume, metachronous high volume, and metachronous low volume) is needed. Arguably, over the next several years, particularly until reliable biomarkers become available, this will be how most clinicians implement treatment for mHSPC patients in North America.
Synchronous High Volume mHSPC
Based on the results from PEACE-118 and ARASENS10, as well as subgroup analysis from ENZAMET3, it appears that this patient cohort, particularly those who are chemotherapy-fit, are most likely to benefit from triplet therapy with docetaxel + androgen receptor pathway inhibitor (ARPI) + ADT.
ADT + Docetaxel + AbirateroneThe PEACE-1 trial18 employed a 2x2 design to assess, (separately and combined) the impact of the addition of abiraterone + prednisone +/- radiation therapy to standard of care therapy in men with de novo mHSPC. Among patients with high volume disease, the addition of abiraterone + prednisone to standard of care resulted in a 53% improvement in rPFS with a median rPFS of 1.6 years in the standard of care arm and 4.1 years in the standard of care plus abiraterone + prednisone arm (HR: 0.47, 95% CI: 0.36 to 0.60). The addition of abiraterone + prednisone to standard of care in patients with low volume disease still resulted in a 42% improvement in rPFS with median rPFS of 2.7 years on the standard of care arm versus not yet reached in the standard of care plus abiraterone + prednisone + ADT arm (HR: 0.58, 95%: CI 0.39 to 0.87). With regards to overall survival in patients with a de novo presentation, a benefit was seen mainly in those with high-volume disease (median overall survival 5.1 versus 3.6 years; HR: 0.77, 95% CI: 0.62 to 0.96), with a marginal, non-significant improvement in those low volume de novo disease (median overall survival not reached; HR: 0.93, 95% CI: 0.69 to 1.28). The overall survival data is immature for the low volume patients due to a small number of events.
Notably, 81% of patients in the ADT plus docetaxel standard of care control arm subsequently received a next generation hormonal therapy at the time of disease progression. This suggests that early intensification with the addition of abiraterone + prednisone to standard of care therapy results in improvement in rPFS and OS compared to sequential therapy.
ADT + Docetaxel + Darolutamide
The ARASENS trial evaluated the addition of darolutamide to standard of care therapy consisting of ADT + docetaxel versus ADT + docetaxel alone.10 Darolutamide + ADT + docetaxel prolonged overall survival for high volume mHSPC (HR 0.69, 95% CI 0.57-0.82).19
Figure 7: Overall survival of darolutamide + ADT + docetaxel vs ADT + docetaxel for high volume disease patients in the ARASENS trial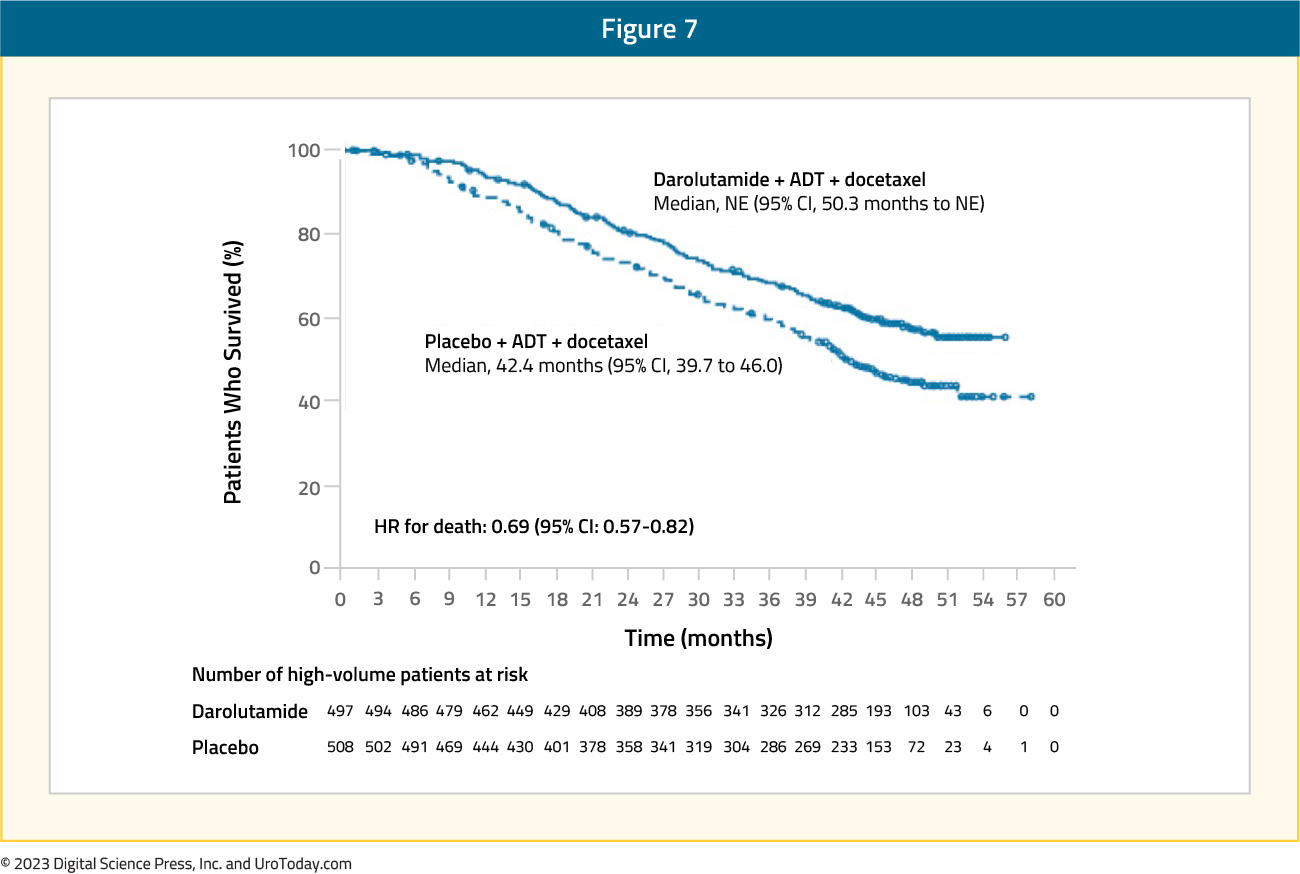
ADT + Enzalutamide vs ADT
While the ENZAMET trial was designed to compare the combination of ADT + enzalutamide versus ADT + standard nonsteroidal antiandrogen, the study design allowed for previous/concurrent use of docetaxel. In this trial, six cycles of docetaxel were given to 65% of patients in the enzalutamide group versus 76% in the standard of care group. Updated results of the ENZAMET trial were published in 20234, with survival outcomes stratified by disease volume (high versus low) and presentation (synchronous versus metachronous). Based on these subgroup analyses, patients with synchronous, high-volume mHSPC had a clinical benefit, albeit not statistically significant, for ADT + enzalutamide versus ADT + standard nonsteroidal antiandrogen whether they were planned to have docetaxel (HR: 0.79, 95% CI: 0.57 to 1.10) or in the intention to treat analysis (HR: 0.70, 95% CI: 0.47 to 1.04).
Figure 8: Overall survival in ENZAMET for patients with synchronous high volume mHSPC
Results from these three trials provide strong evidence to support the use of a triplet regimen approach in patients with synchronous, high volume mHSPC. It bears note, however, that routine use of docetaxel may not be feasible in patients with contraindications to taxane therapy, including poor performance status, blood dyscrasias, and peripheral neuropathy. Such patients would likely benefit from ARPI addition to standard ADT.
Synchronous Low Volume mHSPC
ADT + Docetaxel + ARATFor patients with low volume disease, there appears to be a potential late clinical benefit to triplet therapy of ADT + docetaxel + darolutamide, however, with few events and additional follow-up time likely required, there is no statistically significant benefit at this point (HR: 0.68, 95% CI: 0.41 to 1.13)
Figure 9: Overall survival of darolutamide + ADT + docetaxel vs ADT + docetaxel for low volume disease patients in ARASENS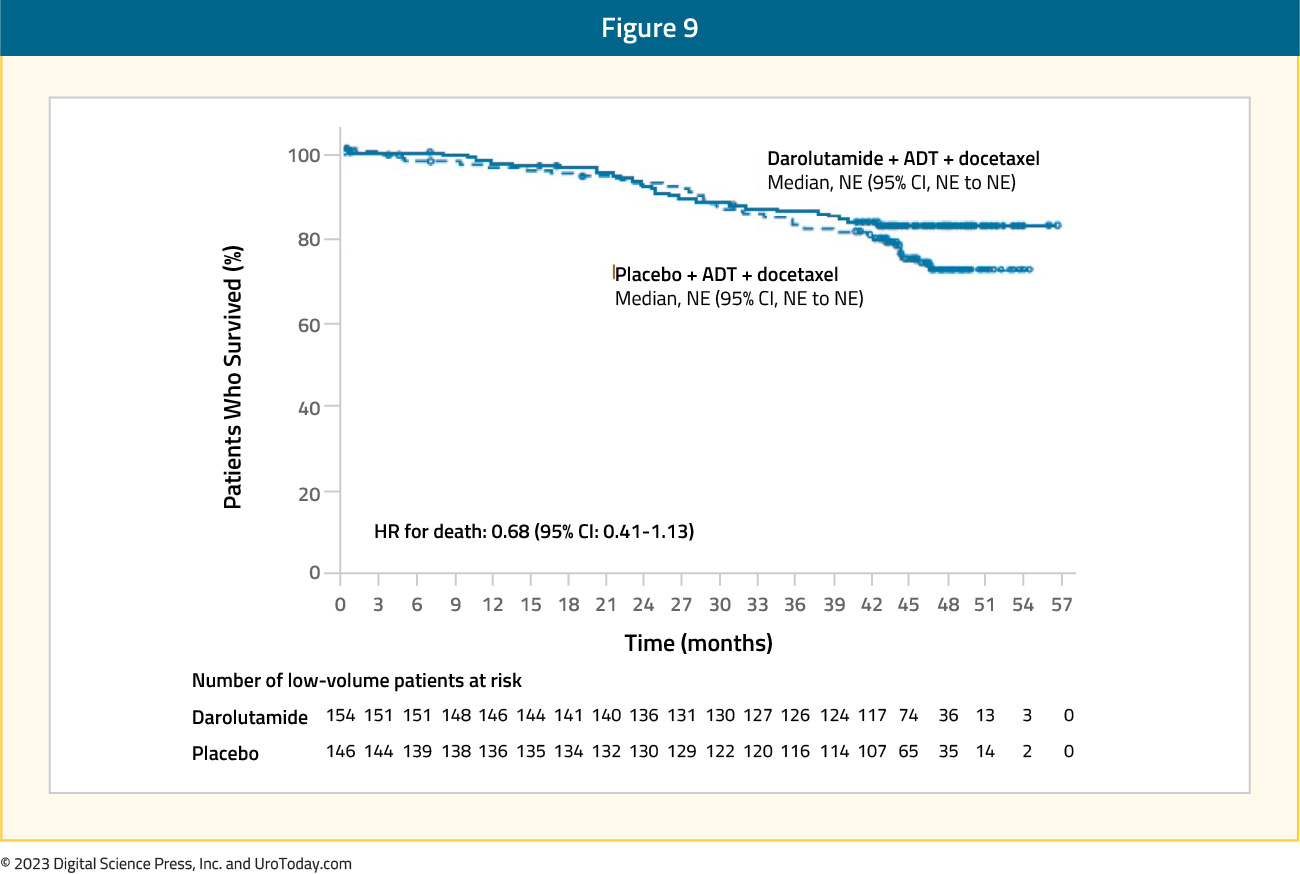
Similarly, for patients treated in the ENZAMET trial with low volume synchronous disease, there was no benefit for ADT + enzalutamide versus ADT + standard nonsteroidal antiandrogen for those planned for docetaxel (HR: 0.57, 95% CI: 0.29 to 1.12).
Figure 10: Overall survival of enzalutamide + ADT + docetaxel vs ADT + docetaxel + non-steroidal anti-androgen for synchronous low volume disease patients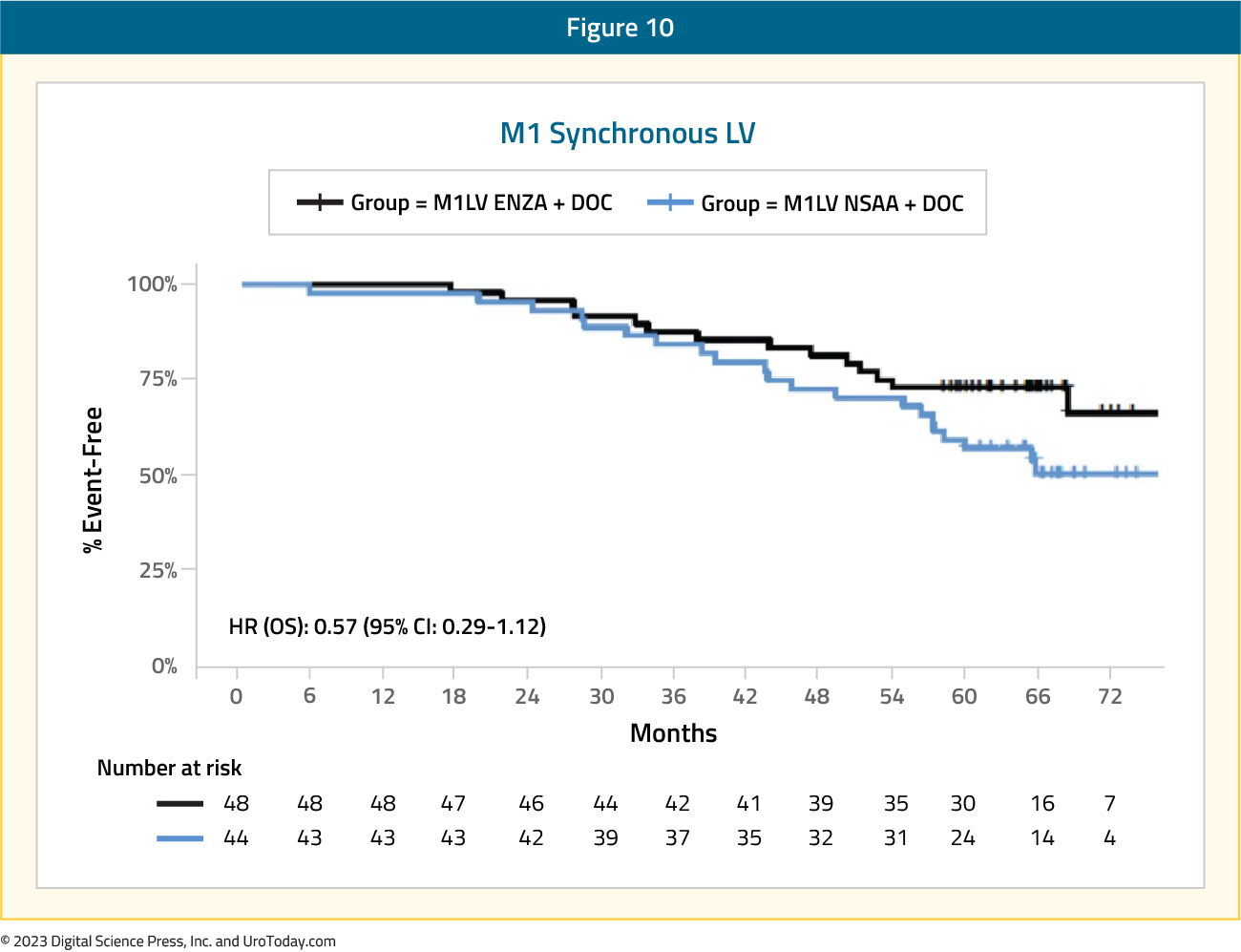
ARATs + ADT
There is consistent evidence across all major published phase III trials to support an overall survival benefit to the addition of an ARPI to ADT in patients with synchronous low-volume disease. This is reflected, as follows:
- LATITUDE (abiraterone + ADT versus ADT alone; all de novo): HR 0.72, 95% CI 0.47 to 1.109
- STAMPEDE (abiraterone + ADT versus ADT alone; >90% de novo): HR 0.64, (95% CI 0.42 to 0.96)10
- TITAN (apalutamide + ADT versus ADT alone; 10% prior docetaxel): HR 0.52, 95% CI 0.35 to 0.7911
- ENZAMET (enzalutamide + ADT versus non-steroidal antiandrogen + ADT): HR 0.58, 95% CI 0.32 to 1.04 4
- ARCHES (enzalutamide + ADT versus ADT alone; 18% prior docetaxel): HR 0.66, 95% CI 0.43 to 1.0312
As such, ARPI addition to ADT has become the backbone of any treatment approach in patients with synchronous, low volume prostate cancer.
Primary Radiotherapy for synchronous, low volume mHSPC patients
Beyond systemic treatment intensification, local prostate-directed therapy may allow for local treatment intensification. While a surgical approach using radical prostatectomy has been described, high quality data are limited to radiotherapy. Of note, the SWOG 1802 trial is accruing patients with a surgical arm in the setting of mHSPC to further assess the impact of cytoreductive prostatectomy in this disease space. Three trials to date have evaluated the role of local radiotherapy in the prostate in patients with mHSPC.
STAMPEDE (Arm H) was an open label, randomized controlled phase III trial of 2,061 men at 117 hospitals across Switzerland and the UK.11 This trial randomized patients with de novo mHSPC in a 1:1 fashion to standard of care + radiotherapy or standard of care alone. Men allocated to radiotherapy received either a daily (55 Gy in 20 fractions over 4 weeks) or weekly (36 Gy in six fractions over 6 weeks) schedule that was nominated before randomization. The primary outcome of this trial was overall survival. Subgroup analysis by metastatic volume (CHAARTED criteria) was planned a priori. Median follow up for STAMPEDE Arm H was 37 months, median patient age was 68 years, and median PSA was 97 ng/ml. 18% of patients received early docetaxel. In the overall cohort, radiotherapy improved failure-free survival (HR: 0.76, 95% CI:0.68 to 0.84) but not overall survival (HR: 0.92, 95% CI: 0.80 to 1.06). However, when stratified by metastatic burden, overall survival benefits were seen in the low volume group (HR: 0.68, 95% CI: 0.52 to 0.90) with restricted mean survival time improved by 3.6 months from 45.4 to 49.1.11
Figure 11: Overall survival in low metastatic burden patients with mHSPC and radiotherapy to the prostate primary in STAMPEDE Arm H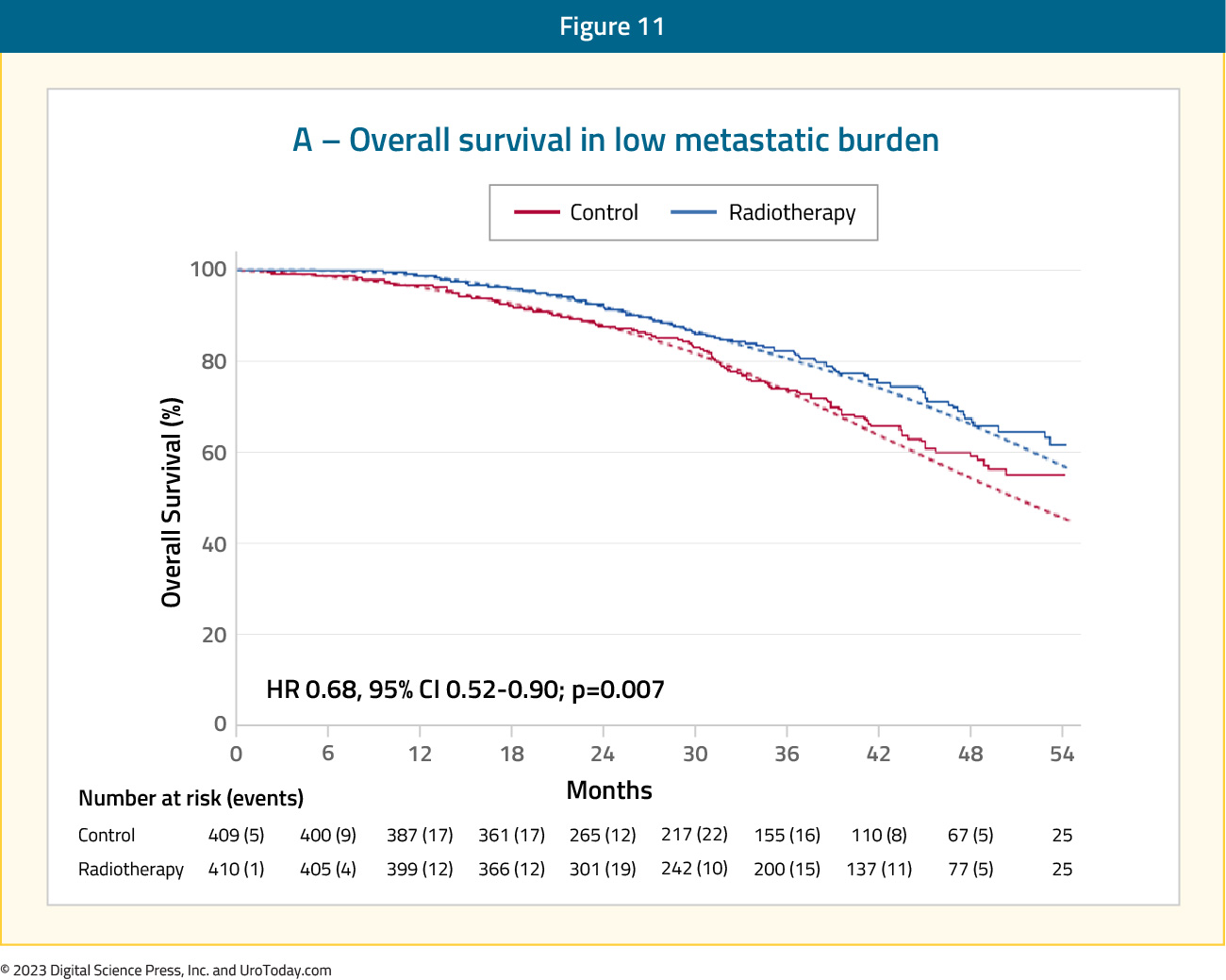
HORRAD was a multicenter prospective randomized clinical trial of 432 patients with previously untreated, de novo mHSPC at 28 centers across The Netherlands between November 2004 and September 2014. 20 All eligible patients had a PSA >20 ng/ml and documented bone metastases on bone scan. Patients were randomized in a 1:1 fashion to either ADT with EBRT or ADT alone, with a primary endpoint of overall survival. The median PSA was 142 ng/mL and over a median follow up of 47 months, the median overall survival was non-significantly different at 45 months in the radiotherapy + ADT arm compared to 43 months in ADT alone arm (HR: 0.90, 95% CI: 0.70 to 1.14).
Results of the efficacy and safety of prostate radiotherapy for patients with low volume, de novo mHSPC from the PEACE-1 trial were recently presented at ASCO 2023. The addition of prostate radiotherapy to standard of care + abiraterone was associated with significant rPFS benefits (median 7.5 versus 4.4 years, p=0.02). Conversely, addition of radiotherapy to standard of care therapy alone was not associated with rPFS benefits (median 2.6 versus 3.0 years; HR: 1.11, 95% CI: 0.67 to 1.84, p=0.61).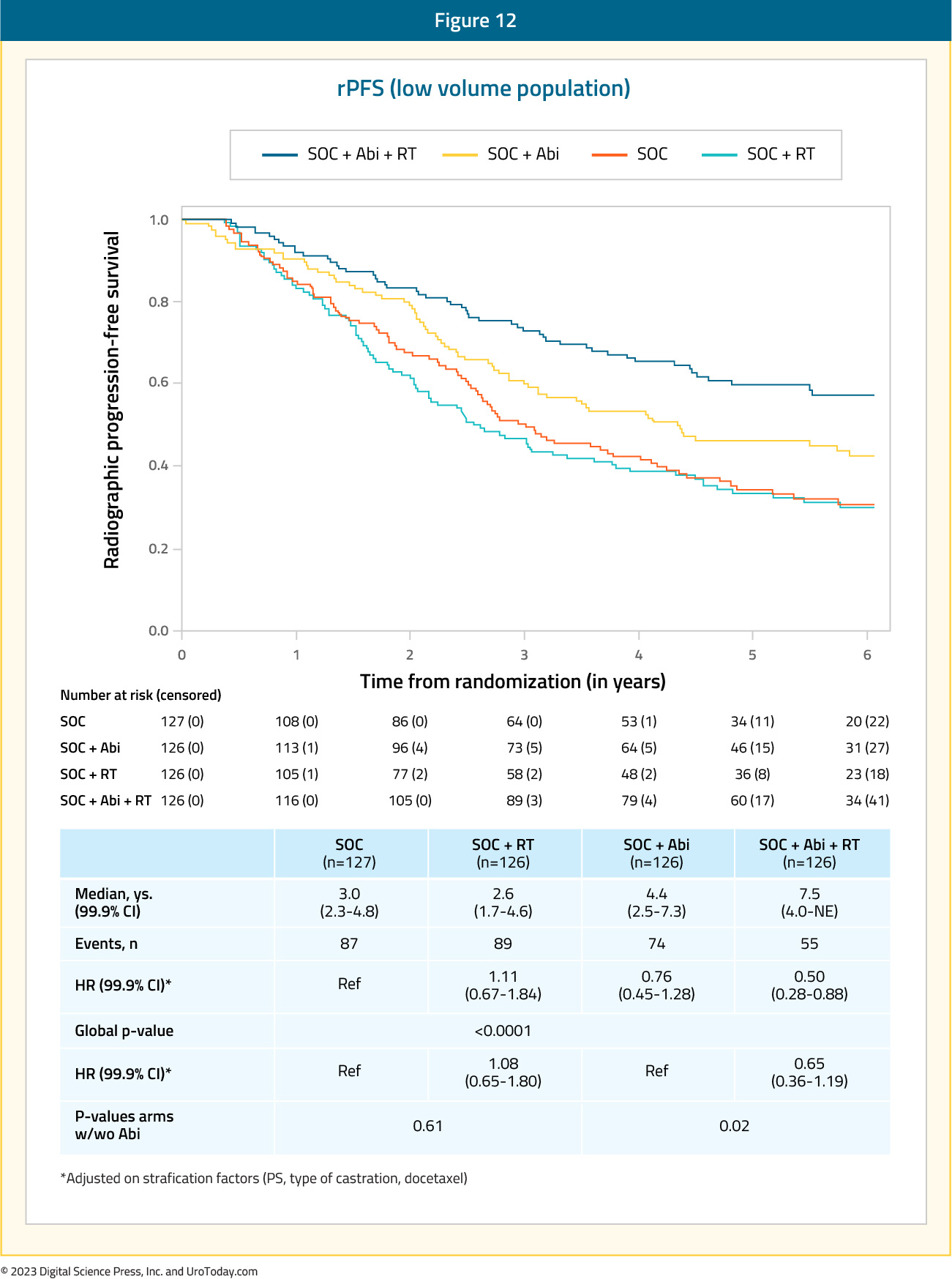
The addition of prostate radiotherapy to either standard of care alone or standard of care therapy + abiraterone was not associated with overall survival improvements. In the standard of care + abiraterone arms, addition of prostate radiotherapy was associated with modest, non-significant OS benefits (HR: 0.77, 95% CI: 0.51 to 1.16, p=0.21). Similarly, addition of prostate radiotherapy to standard of care alone did not improve overall survival (HR: 1.18, 95% CI: 0.81 to 1.71, p=0.39).
Interestingly, addition of prostate radiotherapy to standard of care +/- abiraterone in the low-volume cohort was associated with significant improvements in the time to serious genitourinary events (p=0.0006). This overall benefit was consistent irrespective of whether patients had prostate radiotherapy added to standard of care + abiraterone (p=0.003) or standard of care therapy alone (p=0.048).
The majority of patients with synchronous, low volume mHSPC benefit from early systemic treatment intensification with ARPI addition to ADT. Such patients should also be offered primary radiotherapy to the prostate gland in the appropriate clinical settings.
Metachronous High Volume mHSPC
Docetaxel + ARPI + ADTGiven that the PEACE-1 trial included patients with de novo mHSPC only, ARASENS and ENZAMET provide the available data to assess the benefit of triplet therapy in this mHSPC subgroup. In ARASENS, the overall survival for patients with high volume mHSPC had a prespecified subgroup analysis for assessing recurrent (metachronous) disease (n =117) with a clinical benefit, but no statistically significant benefit (HR: 0.70, 95% CI: 0.39 to 1.24). In the ENZAMET trial, there was no benefit to treatment intensification for triplet therapy (HR: 1.18, 95% CI: 0.66 to 2.11).
Figure 14: Overall survival of enzalutamide + ADT + docetaxel vs ADT + docetaxel + non-steroidal anti-androgen for metachronous high volume disease patients in ENZAMET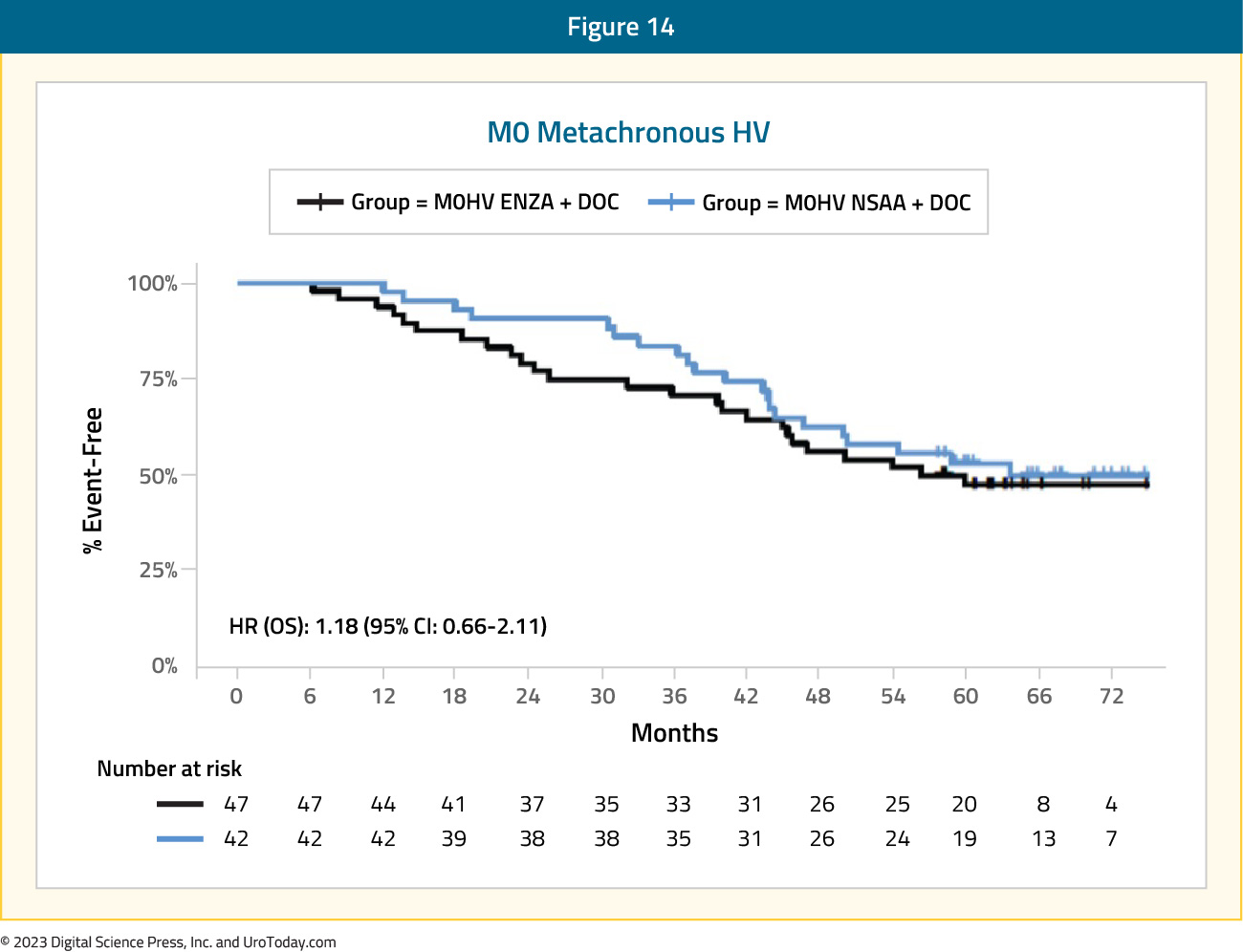
When considering patients with mHSPC along a risk continuum, from good risk (metachronous low volume: 8-year median overall survival with ADT alone) to poor risk (synchronous high volume: 3-year median overall survival with ADT alone), patients with synchronous low volume and metachronous high volume (median overall survival: 4.5 years with ADT alone) mHSPC may both be considered as intermediate risk disease. As such, the clinical treatment approach for these two subgroups has seen significant overlap. ARPI + ADT have similarly served as the backbone for treatment of these patients. Patients with metachronous, high volume mHSPC have historically accounted for only a small proportion of patients in the published phase III trials, and as such, post-hoc analyses have been underpowered for evaluating this subgroup. Results from the ARCHES trial have demonstrated a 23% decreased hazard of overall mortality in this subgroup with addition of enzalutamide to ADT (HR: 0.77, 95% CI: 0.39 to 1.50).5 Similar results were found in the ENZAMET trial (HR: 0.73, 95% CI: 0.37 to 1.44).
Results from the STOPCAP M1 collaborative meta-analysis of individual patient data from GETUG-15, STAMPEDE, and CHAARTED demonstrated that docetaxel addition to ADT in patients with metachronous, high volume prostate cancer is associated with significant improvements in overall survival (HR: 0.64, 95% CI: 0.42 to 0.99),21 which was consistent on follow-up analyses.22
Given the relatively increased toxicity with taxanes, along with a subset of patients being “chemotherapy unfit”, it appears that doublet therapy with an ARPI + ADT is the favored treatment approach in patients with metachronous high volume disease, with docetaxel reserved for select patients with higher volume of disease.
Metachronous Low Volume mHSPC
Docetaxel + ARPI + ADTSimilar to metachronous high volume patients, the subgroup analyses of triplet therapy trials assessing metachronous low volume mHSPC patients remain limited. In ARASENS, metachronous low volume disease patients (n = 51) had too few overall mortality events to provide adequate samples size for powered analyses. For the ENZAMET trial, the HR for metachronous low volume patients was 0.64 (95% CI: 0.18 to 2.28).
Figure 15: Overall survival of enzalutamide + ADT + docetaxel vs ADT + docetaxel + non-steroidal anti-androgen for metachronous low volume disease patients in ENZAMET
ARPI + ADT
Subgroup analyses have consistently demonstrated an overall survival benefit to ARPI addition in patients with low volume mHSPC. Results from the ARCHES trial demonstrated a 37% improved hazard of overall survival with enzalutamide addition to ADT in patients with metachronous low volume mHSPC (HR: 0.63, 95% CI: 0.26 to 1.54). From the ENZAMET trial, patients with metachronous low volume disease had a clinical and statistically significant benefit (HR of 0.47, 95% CI: 0.28 to 0.79).
Docetaxel + ADTImportantly, there is consistent evidence against the use of docetaxel in this mHSPC subgroup. Results from the CHAARTED trial demonstrated a minimal overall survival in this subgroup (HR: 0.77, 95% CI: 0.51 to 1.18). Furthermore, results from the STOPCAP meta-analysis using CHAARTED and GETUG-AFU15 data demonstrated no overall survival benefit to docetaxel addition (HR: 1.07, 95% CI: 0.75 to 1.54).
Table 1: A summary of overall survival by volume and timing of metastases from the key registration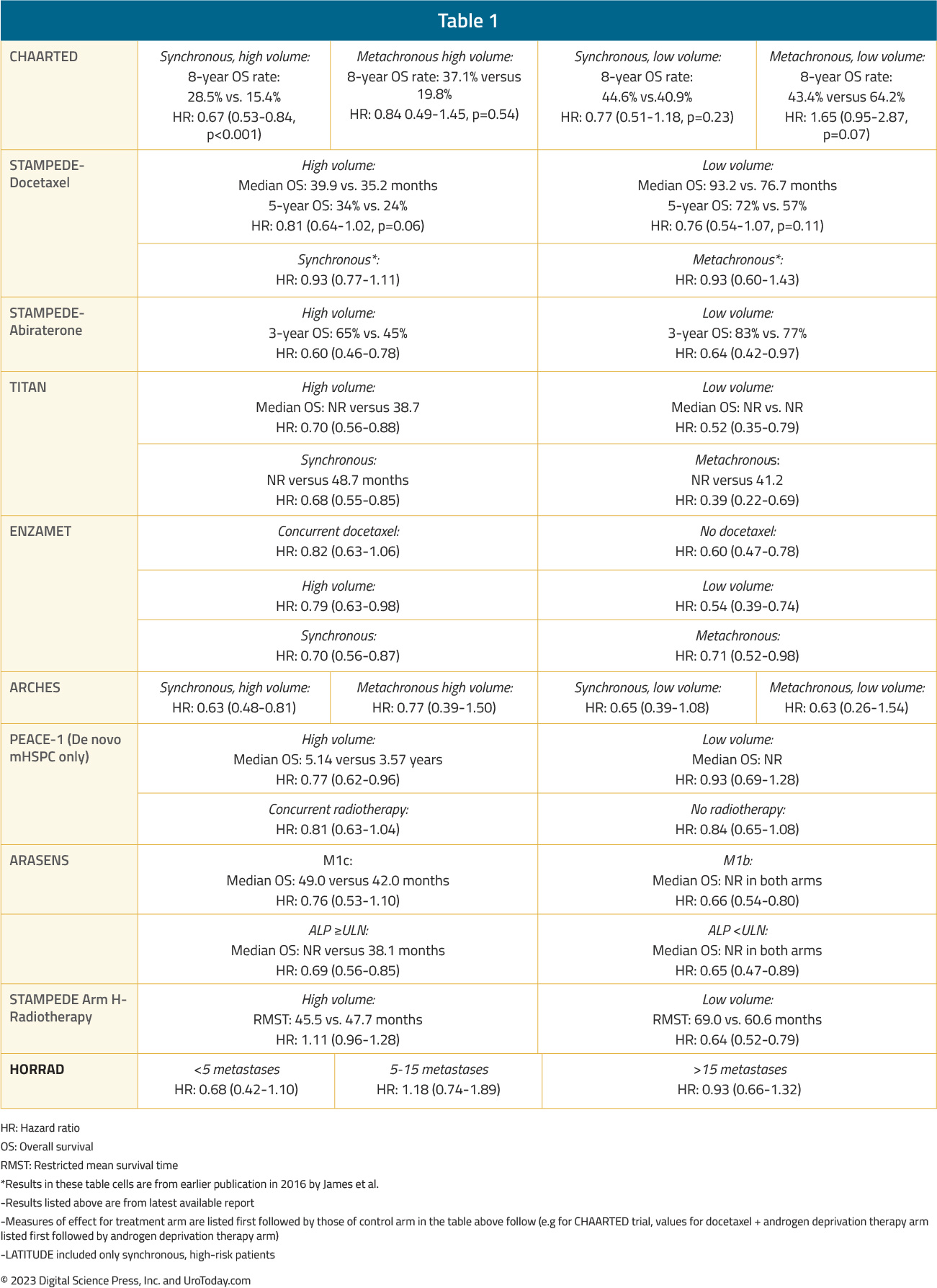
Conclusions
Although there appears to be increasing utilization of treatment intensification in the real-world setting, less than half of mHSPC patients receive guideline concordant care. While there may be altruistic reasons to avoid treatment intensification secondary to concerns for patient financial toxicity or concerns for the tolerability of these agents, the proven survival benefit conferred by this treatment paradigm should make this approach the clear standard of care. Based on the current evidence, it appears that patients with synchronous, high volume mHSPC benefit from early treatment intensification with triplet therapy in the form of both an ARPI and docetaxel, whereas the remaining mHSPC subgroups benefit most from doublet therapy with ARPI addition to ADT. Radiotherapy to the prostate is also associated with improved overall survival in mHSPC patients with synchronous, low-volume disease and should be considered in these cases.Related Content: New Pathways for Treating Metastatic Castration-Resistant Prostate Cancer (mCRPC)
Published November 2023
Part of an Independent Medical Education Initiative Supported by LOXO@Lilly
- Written by: Zachary Klaassen, MD MSc Georgia Cancer Center Wellstar MCG Health Augusta, Georgia and Rashid Sayyid, MD MSc University of Toronto Toronto, ON
- References:
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2019;381(1):13-24.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39(20):2294-2303.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121-131.
- Sweeney CJ, Martin AJ, Stockler MR, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(4):323-334.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974-2986.
- Armstrong AJ, Iguchi T, Azad AA, et al. The Efficacy of Enzalutamide plus Androgen Deprivation Therapy in Oligometastatic Hormone-sensitive Prostate Cancer: A Post Hoc Analysis of ARCHES. Eur Urol. 2023;84(2):229-241.
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352-360.
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686-700.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022;386(12):1132-1142.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Med. 2022;19(6):e1003998.
- Ryan CJ, Ke X, Lafeuille MH, et al. Management of Patients with Metastatic Castration-Sensitive Prostate Cancer in the Real-World Setting in the United States. J Urol. 2021;206(6):1420-1429.
- Freedland SJ, Sandin R, Sah J, et al. Treatment patterns and survival in metastatic castration-sensitive prostate cancer in the US Veterans Health Administration. Cancer Med. 2021;10(23):8570-8580.
- Wallis CJD, Malone S, Cagiannos I, et al. Real-World Use of Androgen-Deprivation Therapy: Intensification Among Older Canadian Men With de Novo Metastatic Prostate Cancer. JNCI Cancer Spectr. 2021;5(6).
- Deek MP, Van der Eecken K, Phillips R, et al. The Mutational Landscape of Metastatic Castration-sensitive Prostate Cancer: The Spectrum Theory Revisited. Eur Urol. 2021;80(5):632-640.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737-746.
- Fizazi K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 x 2 factorial design. Lancet. 2022;399(10336):1695-1707.
- Hussain M, Tombal B, Saad F, et al. Darolutamide Plus Androgen-Deprivation Therapy and Docetaxel in Metastatic Hormone-Sensitive Prostate Cancer by Disease Volume and Risk Subgroups in the Phase III ARASENS Trial. J Clin Oncol. 2023;41(20):3595-3607.
- Boeve LMS, Hulshof M, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
- Vale CL, Burdett S, Rydzewska LHM, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17(2):243-256.
- Vale CL, Fisher DJ, Godolphin PJ, et al. Which patients with metastatic hormone-sensitive prostate cancer benefit from docetaxel: a systematic review and meta-analysis of individual participant data from randomised trials. Lancet Oncol. 2023;24(7):783-797.
New Cancer Bundle from CMS: the Enhancing Oncology Model
Emerging Therapeutic Options for Low-Grade Non-Invasive Bladder Cancer: Primary Chemoablation
Introduction
Bladder cancer remains the sixth most commonly diagnosed cancer in the United States, with an estimate of 82,290 incident cases in 2023.1 At diagnosis, approximately 75% of patients present with non-muscle invasive disease, with significant clinical heterogeneity observed within this disease group.2,3 Patients with initial low-grade Ta disease (i.e., confined to the mucosal lining) represent a unique patient cohort given their favorable long-term oncologic outcomes, given that they are more likely to recur than progress to life-threatening disease.4
- Written by: Rashid K. Sayyid, MD, MSc University of Toronto Toronto, ON and Zachary Klaassen, MD, MSc Medical College of Georgia Augusta, Georgia, USA
- References:
- American Cancer Society. Key Statistics for Bladder Cancer.
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur Urol 2019; 76(5):639-57.
- Monteiro LL, Witjes JA, Agarwal PK, et al. ICUD-SIU International Consultation on Bladder Cancer 2017: management of non-muscle invasive bladder cancer. World J Urol 2019; 37(1):51-60.
- Hernandez V, Llorente C, de la Pena E, et al. Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer. Urol Oncol 2016; 34(4):165.e19-23.
- Mossanen M, Gore JL. The Burden of Bladder Cancer Care – Direct and Indirect Costs. Curr Opin Urol 2014; 24(5):487-91.
- Zhou Z, Zhao S, Lu Y, et al. Meta-analysis of efficacy and safety of continuous saline bladder irrigation compared with intravesical chemotherapy after transurethral resection of bladder tumors. World J Urol, 2019; 37(6):1075.
- Sylvester RJ, et al. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? Eur Urol 2016; 69(2): 231-44.
- Perlis N, Zlotta AR, Beyene J, et al. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. Eur Urol 2013; 64(3):421-30.
- Messing EM, Tangen CM, Lerner SP, et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. JAMA 2018; 319(18):1880-8.
- Arends TJH, Nativ O, Maffezzini M, et al. Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guérin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non-Muscle-invasive Bladder Cancer. Eur Urol 2016; 69(6):1046-52.
- Arends TJH, van der Heijdem AG, Witjes JA. Combined chemohyperthermia: 10-year single center experience in 160 patients with nonmuscle invasive bladder cancer. J Urol 2014; 192(3):708-13.
- Shelley MD, Kynaston H, Court J, et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int 2001; 88(3):209-16.
- Han RF, Pan JG. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology 2006; 67(6):1216-23.
- Shelley MD, Wilt TJ, Court J, et al. Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int 2004; 93(4):485-90.
- Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 2003; 169(1):90-5.
- Popert RJ, Goodall J, Coptcoat MJ, et al. Superficial bladder cancer: the response of a marker tumour to a single intravesical instillation of epirubicin. Br J Urol 1994; 74(2):195-9.
- Mostafid AH, Porta N, Cresswell J, et al. CALIBER: a phase II randomized feasibility trial of chemoablation with mitomycin‐C vs surgical management in low‐risk non‐muscle‐invasive bladder cancer. BJU Int 2020; 125(6):817-26.
- Lindgren MS, Bue P, Azawi N, et al. The DaBlaCa-13 Study: Short-term, Intensive Chemoresection Versus Standard Adjuvant Intravesical Instillations in Non-muscle-invasive Bladder Cancer-A Randomised Controlled Trial. Eur Urol 2020; 78(6):856-62.
- Kleinmann N, Matin SF, Pierorazio PM, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol 2020; 21(6):776-85.
- FDA approves mitomycin for low-grade upper tract urothelial cancer.
- Chevli KK, Shore ND, Trainer A, et al. Primary Chemoablation of Low-Grade Intermediate-Risk Nonmuscle-Invasive Bladder Cancer Using UGN-102, a Mitomycin-Containing Reverse Thermal Gel (Optima II): A Phase 2b, Open-Label, Single-Arm Trial. J Urol 2022; 207(1):61-9.
- Prasad SM, Huang WC, Shore ND, et al. Treatment of Low-grade Intermediate-risk Nonmuscle-invasive Bladder Cancer With UGN-102 ± Transurethral Resection of Bladder Tumor Compared to Transurethral Resection of Bladder Tumor Monotherapy: A Randomized, Controlled, Phase 3 Trial (ATLAS). J Urol 2023; 101097JU0000000000003645.
National Cancer Drug Shortages: Policy Response and Potential Solutions
The Treatment Landscape of Metastatic Urothelial Carcinoma: Second-Line Systemic Therapy
Introduction
For patients with metastatic urothelial carcinoma, first line therapy for cisplatin eligible patients remains platinum-based chemotherapy1 (followed by maintenance avelumab2), whereas those that are cisplatin ineligible may receive gemcitabine + carboplatin3 (followed by maintenance avelumab2), pembrolizumab,4 or pembrolizumab + enfortumab vedotin.5 Unfortunately, ~50% of patients will eventually have disease progression following first line therapy, which has historically been associated with a dismal prognosis.
- Written by: Zachary Klaassen, MD, MSc Associate Professor of Urology Urologic Oncologist Medical College of Georgia, Georgia Cancer Center Augusta, GA and Rashid Sayyid, MD, MSc Urologic Oncology Fellow University of Toronto Toronto, Ontario, Canada
- References:
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18: 3068-3077.
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383: 1218-1230.
- De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30: 191-199.
- Powles T, Csoszi T, Ozguroglu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22: 931-945.
- Hoimes CJ, Flaig TW, Milowsky MI, et al. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. J Clin Oncol. 2023;41: 22-31.
- Vaughn DJ, Broome CM, Hussain M, Gutheil JC, Markowitz AB. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol. 2002;20: 937-940.
- Galsky MD, Mironov S, Iasonos A, Scattergood J, Boyle MG, Bajorin DF. Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs. 2007;25: 265-270.
- McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol. 1997;15: 1853-1857.
- Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27: 4454-4461.
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376: 1015-1026.
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18: 312-322.
- Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol. 2017;35: 2117-2124.
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019;381: 338-348.
- Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15: 25-41.
- Sideris S, Aoun F, Zanaty M, et al. Efficacy of weekly paclitaxel treatment as a single agent chemotherapy following first-line cisplatin treatment in urothelial bladder cancer. Mol Clin Oncol. 2016;4: 1063-1067.
- Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. 2001;19: 2638-2646.
- Yu EY, Petrylak DP, O'Donnell PH, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV‑201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021;22: 872-882.
National Cancer Drug Shortages: History and Current Status
BCG-unresponsive Non-Muscle Invasive Bladder Cancer: Immune Checkpoint Inhibitors
Introduction
Intravesical Bacillus Calmette-Guerin (BCG) currently remains the standard-of-care, guideline recommended treatment of choice in the adjuvant setting for intermediate- and high-risk non-muscle invasive bladder cancer (NMIBC) due to its ability to reduce the risk of disease recurrence and, more importantly, disease progression.1-3 However, despite adequate BCG treatment, defined as receipt of at least five doses of the initial six-dose induction course and at least 2/3 maintenance doses or at least 2/6 doses of the second induction course, up to 50% of such patients will develop a BCG-refractory, relapsing, or failure state.4 Currently, radical cystectomy remains the gold standard approach in this setting.1 However, many patients are either unfit or refuse cystectomy. As such, bladder-sparing approaches in this setting are of utmost importance.
- Written by: Rashid K. Sayyid, MD, MSc University of Toronto Toronto, ON and Zachary Klaassen, MD, MSc Medical College of Georgia Augusta, Georgia, USA
- References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Schmidt S, Kunath F, Coles B, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Review. 2020;1(1):CD011935.
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 Update. Eur Urol. 2019;76(5):639–57.
- Steinberg R, Bahnson R, Brosman S, et al. Efficacy and Safety of Valrubicin for the Treatment of Bacillus Calmette-Guerin Refractory Carcinoma in Situ of the Bladder. J Urol. 200;163(3):761-7.
- Bacillus Calmette-Guérin-unresponsive nonmuscle invasive bladder cancer: developing drugs and biologics for treatment guidance for industry. 2018.
- FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer.
- FDA D.I.S.C.O. Burst Edition: FDA approval of Adstiladrin (nadofaragene firadenovec-vncg) for patients with high-risk Bacillus Calmette-Guérin unresponsive non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors.
- Kates M, Matoso A, Choi W, et al. Adaptive Immune Resistance to Intravesical BCG in Non–Muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin Cancer Res. 2020;26(4):882-91.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22(7):919-30.
- Necchi A, Roumiguié M, Esen AA, et al. Pembrolizumab (pembro) monotherapy for patients (pts) with high-risk non–muscle-invasive bladder cancer (HR NMIBC) unresponsive to bacillus Calmette–Guérin (BCG): Results from cohort B of the phase 2 KEYNOTE-057 trial. J Clin Oncol. 2023;41(Supp 6):LBA442.
- Alanee S, Sana S, El-Zawahry A, et al. Phase I trial of intravesical Bacillus Calmette-Guérin combined with intravenous pembrolizumab in recurrent or persistent high-grade non-muscle-invasive bladder cancer after previous Bacillus Calmette-Guérin treatment. World J Urol. 2021;39(10):3807-13.
- Meghani K, Cooley LF, Choy B, et al. First-in-human Intravesical Delivery of Pembrolizumab Identifies Immune Activation in Bladder Cancer Unresponsive to Bacillus Calmette-Guérin. Eur Urol. 2022;82(6):602-10.
- Li R, Sexton WJ, Dhillon J, et al. A Phase 2 Study of Durvalumab for Bacillus Calmette-Guerin (BCG) Unresponsive Urothelial Carcinoma In Situ of the Bladder. Clin Cancer Res. 2023.
- Kowalski M, Guindon J, Brazas L, et al. A phase II study of oportuzumab monatox: an immunotoxin therapy for patients with noninvasive urothelial carcinoma in situ previously treated with bacillus Calmette-Guérin. J Urol. 2012;188(5):1712-8.
- Gurram S, Bellfield S, Dolan R, et al. PD09-04 Interim analysis of a phase I single-arm study of the combination of durvalumab (Medi4736) and vicinium (Oportuzumab Monatox, Vb4-845) in subjects with high-grade non-muscle-invasive bladder cancer previously treated with Bacillus Calmette-Guerin (Bcg). J Urol. 2021;206(Suppl 3):e120.
- Black PC, Tangen C, Singh P, et al. Phase II trial of atezolizumab in BCG-unresponsive non-muscle invasive bladder cancer: SWOG S1605 (NCT #02844816). J Clin Oncol;2020(Suppl):5022.
- Shore ND, Powles T, Bedke J, et al. A phase 3 study of the subcutaneous programmed cell death protein 1 inhibitor sasanlimab as single agent for patients with bacillus Calmette-Guérin, unresponsive high-risk, non-muscle invasive bladder cancer: CREST Study Cohort B. J Clin Oncol. 2022;40(Suppl 16):40.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021;384:2102-14.
- Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499-505.
- Hudolin T, Mengus C, Coulot J, et al. Expression of Indoleamine 2,3-Dioxygenase Gene is a feature of poorly differentiated non-muscle-invasive urothelial cell bladder carcinomas. Anticancer Res. 2017;37(3):1375-80.
- Tabernero, et al. BMS-986205, an indoleamine 2,3-dioxygenase 1 inhibitor (IDO1i), in combination with nivolumab (NIVO): Updated safety across all tumor cohorts and efficacy in pts with advanced bladder cancer (advBC). J Clin Oncol. 2018;36(Suppl 15):4512.
Prostate Radiotherapy for De Novo, Low Volume Metastatic Hormone Sensitive Prostate Cancer: Is There Benefit?
- Written by: Rashid Sayyid, MD, MSc and Zachary Klaassen, MD, MSc
- References:
- Schaeffer EM, Srinivas S, Adra A, et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J Natl Compr Canc Netw. 2022;20(12):1288-1298.
- Bossi A, Foulon S, Maldonado X, et al. Prostate irradiation in men with de novo, low-volume, metastatic, castration-sensitive prostate cancer (mCSPC): Results of PEACE-1, a phase 3 randomized trial with a 2x2 design. J Clin Oncol. 2023;41(17):Suppl.
- Boeve LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Medicine. 2022;19(6):e1003998.
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373:737-746.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.
- Fizai K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022;399(10336):1695-1707.
BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: Review of Intravesical Chemotherapy and Photodynamic Therapy
Introduction
Intravesical Bacillus Calmette-Guerin (BCG) remains the current standard-of-care, guideline-recommended treatment of choice in the adjuvant setting for intermediate- and high-risk non-muscle invasive bladder cancer (NMIBC) due to its ability to reduce the risk of disease recurrence and, more importantly, disease progression.1-3 Despite adequate BCG treatment up to 50% of patients will develop a BCG-refractory, relapsing, or failure state.4 Over the last several years, there has been a plethora of data in the BCG unresponsive disease space, leading to FDA approvals for pembrolizumab in January 2020 and nadofaragene firadenovec in December 2022.6 Many of these novel immune-based treatments overcome some of the limitations of older agents in this setting,7 particularly those related to short durations of exposure limiting their efficacy.8 This same concept of extending the intravesical exposure time has recently been extrapolated to the intravesical chemotherapeutic treatment landscape, where we have witnessed the emergence of novel agents with prolonged mechanisms of action and alternate methods of administration. In this Center of Excellence article, we will discuss the currently available evidence and ongoing studies for intravesical chemotherapeutic agents and photodynamic therapy in the BCG unresponsive NMIBC disease space.
Gemcitabine + Docetaxel
In 2020, the results of a multicenter, retrospective analysis evaluating sequential gemcitabine plus docetaxel in patients with recurrent NMIBC and a history of intravesical BCG treatment were reported. In this study, all patients received intravesical gemcitabine (1 gm/50 ml sterile water or saline) for 60 to 90 minutes, after which it was drained and intravesical docetaxel (37/5 mg/50 ml saline) was instilled and held for 1-2 hours. To minimize side effects secondary to the irritative effects of gemcitabine (pH 2.5), 1,300 mg of oral sodium bicarbonate was given the evening before and the morning of each instillation to alkalinize the urine. The induction regimen was given once weekly for 6 weeks, followed by monthly maintenance for up to 24 months in the majority of the participating institutions. Surveillance was performed in accordance with institutional/national guidelines.This analysis included 276 patients with a median age of 73 years. The median number of prior BCG courses was 2 (range: 1 to 8), with 38% of patients meeting the BCG unresponsive disease definition. From an efficacy standpoint, the 1- and 2-year recurrence-free survival rates were 60% and 46%, respectively, with similar recurrence rates observed for patients with CIS versus papillary-only disease. The high-grade recurrence-free survival rates in the overall cohort at 1 and 2 years were 65% and 52%, respectively:

Among patients with BCG unresponsive disease, the 2-year high-grade recurrence-free survival rates were 50% and 58% for CIS and papillary disease-alone cases, respectively:

Overall, 43/276 patients (15.6%) underwent a radical cystectomy, at a median follow-up of 11.3 months, and 11 patients (4%) had evidence of progression to muscle invasive disease. The most common side effects were frequency/urgency and dysuria, with 41% of patients reporting symptoms during treatment, but only 9.3% having symptoms that impacted the treatment schedule. There were no reported treatment-related deaths.9
Long-term follow-up of a subset of this cohort from the University of Iowa was recently published in 2023. This analysis included 97 patients (35% BCG unresponsive), with a median follow-up of 49 months. The 3-month complete response rate was 74%, with a median duration of response of 25 months. The high-grade recurrence-free survival rates were:
- 1 year: 60%
- 2 years: 50%
- 5 years: 30%
Device-assisted Administration of Intravesical Chemotherapy
TAR-200TAR-200/gemcitabine (JNJ-17000139) is a novel drug delivery system that allows for the sustained local release of gemcitabine intravesically, relying on an osmotic system as illustrated below:
Evaluation of urine and plasma gemcitabine concentrations over a 7-day period demonstrates the ability of TAR-200 to provide sustained, local delivery of gemcitabine while limiting systemic toxicity. As demonstrated in the figure below, intravesical instillation of gemcitabine (green curve) is associated with a sharp increase/decrease in the gemcitabine urine concentration, which is non-sustained beyond day 1. Conversely, we see an increase in the urinary gemcitabine concentration between days 1 and 3 with TAR-200 (blue curve), followed by a gradual decline with measurable levels detected until at least day 7. Importantly, no gemcitabine is detected in the plasma of patients receiving TAR-200.

SunRISe-1 is a randomized trial of BCG-unresponsive, high-risk NMIBC patients with CIS +/- papillary disease, who did not receive a radical cystectomy. Patients underwent stratified randomization (by presence or absence of concomitant papillary disease) in a 2:1:1 fashion to either:
- Cohort 1: TAR-200 + cetrelimab (PD-1 inhibitor)
- Cohort 2: TAR-200 alone (target sample size 50, currently enrolled: 23)
- Cohort 3: Cetrelimab alone (target sample size 50, currently enrolled: 24)
The median patient age was 70 – 72 years, pure CIS was present in 70% and 65% of patients in the TAR-200 and cetrelimab groups, respectively, and the median total doses of prior BCG were 12 in each arm. From an efficacy standpoint, 73% of patients in the TAR-200 arm achieved a complete response (median duration of response not yet reached), defined based on the results of cystoscopy, centrally assessed urine cytology, and mandated biopsy at weeks 24 and 48. The CR rate in the cetrelimab arm was 38%.
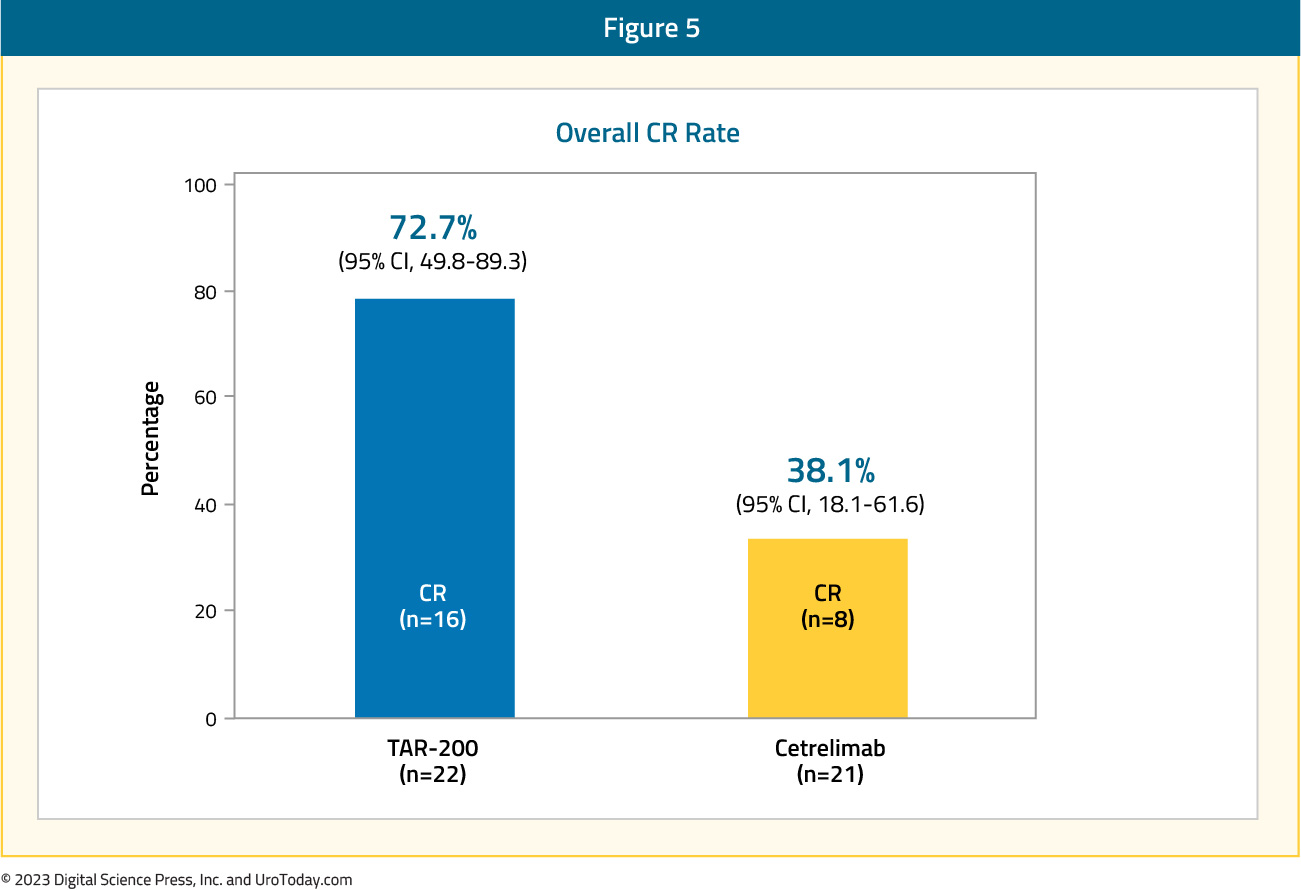
Overall, most adverse events in the TAR-200 group were grade ≤2, with those reported in the cetrelimab group similar to that expected and observed with other PD-1 agents. 9% and 4% of patients discontinued TAR-200 and cetrelimab due to treatment-related adverse events. In each arm, 1 patient had treatment-related serious adverse events and 2 patients had treatment-related grade ≥3 adverse events. No deaths were observed in the study.11

Given these promising findings from the TAR-200 monotherapy arm, Janssen announced that they will be suspending further enrollment to the TAR-200 + cetrelimab arm.
Radiofrequency-induced Thermochemotherapy
In 2009, the results of a multi-institutional analysis of 51 patients from 15 European centers receiving mitomycin combined with intravesical hyperthermia in patients with mainly BCG-failing CIS (35/51) were published. Patients received mitomycin via the Synergo® system SB-TS 101, which uses an intravesical microwave applicator to deliver hyperthermia to the bladder wall via direct irradiation, weekly for 6–8 weeks, followed by 4–6 sessions every 6–8 weeks. Of 49 evaluable patients, there was evidence of a complete CIS response (negative biopsy and cytology) at 3 months in 45 patients (92%). Patients had similar outcomes irrespective of whether they met the BCG failure definition or not. Among the 45 responders, 49% had a recurrence at a median follow-up of 22 months. The most common adverse events were bladder spasms (13.1%), pain (12.7%), and dysuria (6.2%), and were generally mild in severity.12A follow-up analysis of 150 patients who received ≥6 radiofrequency mitomycin instillations (induction and maintenance) was reported in 2018. All included patients had either pathology or cystoscopy plus cytology available at 6 months of follow-up. Of these 150 patients, 50 (33.3%) had BCG-unresponsive disease, with a 6-months complete response rate of 36% in this subgroup. The two-year recurrence-free survival rate was 82.6%, with a three-year cystectomy-free rate of 71.4%. Treatment discontinuation due to adverse events occurred in 13.4% of patients receiving induction treatment and 17.8% of those receiving maintenance instillations.13
Hyperthermic Intravesical Chemotherapy
In 2018, de Joeng et al. reported the results of hyperthermic intravesical chemotherapy (HIVEC) in 52 patients with BCG unresponsive NMIBC. Patients received intravesical instillations of mitomycin (80 mg in 50 mL of distilled water) that were extravesically heated up to 41-43°C and recirculated during 60 minutes at 200 m/min at stable pressure. All instillations were conducted with the Combat BRS system V2.0. The 3-months complete response rate was 75%. By 12 months follow-up, 47% of patients had remained disease-free. The overall median disease-free survival was 17.7 months and was significantly longer in those with papillary disease at 28.8 months versus 17.7 months in those with CIS. Patients in the ‘very high risk’ BCG unresponsive group had the shortest disease-free survival duration at 12.1 months. Any adverse event was reported in 69% of patients, with almost all grade 1-2 in severity, most common of which was urinary frequency/urgency (35%), urinary tract pain (15%), and bladder spasms (7%).14
In 2022, results of the multi-institutional Hyperthermic Chemotherapy registry (HIVEC-E) were published. These included a total of 1,028 patients, with 172 (21%) and 74 (9%) having disease classified as BCG unresponsive and failure, respectively. The 12- and 24-months recurrence-free survival rates for the BCG unresponsive cohort were 78.1% and 57.4%, respectively. Among patients in the BCG unresponsive cohort, the 24-months recurrence-free survival rates were superior for those papillary only disease (64.5% versus 43.6% for those with CIS). Minor and severe adverse events occurred in 26% and 2% of patients, respectively.15
Photodynamic Therapy
TLD-1433 is a novel ruthenium-based photosensitizer that selectively binds to bladder cancer cells. When activated by a 520 nm intravesical laser (TLC-3200) under general anesthesia (90 J/cm2 of laser light), it generates cytotoxic singlet oxygen and radical oxygen species, leading to cell death. PDT is also known to induce an antitumor cascade of immune signaling.16,17
At ASCO GU 2023, Dr. Girish Kulkarni presented the interim results of a phase II trial evaluating this intravesical photo dynamic therapy in patients with BCG unresponsive CIS. At the time of presentation, the trial had enrolled 52 patients (70% pure CIS, 20% CIS + HG T1, 10% CIS + Ta). This was a heavily pre-treated cohort, with 21% having >19 prior BCG instillations. A complete response, defined by a negative cystoscopy and cytology, was observed in 54% of patients. Among the 12 patients who had available follow-up at 450 days, 8 (67%) had a complete response. While 9/52 patients experienced a serious adverse event, none were deemed directly treatment-related per the Data Safety Monitoring Board.18
Enfortumab Vedotin
Enfortumab vedotin (EV) is a Nectin-4 targeted antibody-drug conjugate. Nectin-4 is highly expressed on bladder tumor cells, making it an attractive target for such agents. Systemic use of EV has previously demonstrated an overall survival benefit in the 3rd line setting for patients with locally advanced or metastatic urothelial carcinoma, who had previously received platinum-based therapy and a PD-1 or PD-L1 inhibitor (EV-301).19 Based on its demonstrated benefit in locally advanced/metastatic urothelial carcinoma, EV is currently being evaluated in earlier settings. EV-104 (NCT05014139) is a phase 1, open-label, multicenter, dose-escalation, and dose-expansion study of intravesical EV in adults with high-risk BCG-unresponsive NMIBC (CIS +/- papillary disease) who are ineligible for or refuse radical cystectomy. The primary study objectives are to evaluate the safety and tolerability of intravesical EV and determine the maximum-tolerated and recommended phase II doses of intravesical EV.Similar in concept to BCG and intravesical chemotherapy regimens, the EV-104 treatment regimen will include an induction phase, whereby patients will receive intravesical EV weekly for 6 weeks followed by monthly maintenance for a total of 9 additional EV doses. Patients will undergo cystoscopy and cytology every 3 months and annual upper tract imaging.

The study is currently enrolling in the United States (since January 2022) with additional sites planned in Canada and Europe, including the UK.
Erdafitinib
Erdafitinib is an oral selective pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor that is approved for locally advanced or metastatic urothelial carcinoma in adults with FGFR3/2 alterations who have progressed during or after at least one line of platinum-containing chemotherapy.20 THOR-2 is a multi-cohort, phase II trial of erdafitinib in patients with high-risk NMIBC, with Cohort 2 including patients with BCG-unresponsive CIS (+/- papillary disease) and FGFR3/2 alterations. In this cohort, patients received continuous oral erdafitinib 6 mg once daily.Interim results were presented at ASCO GU 2023 by Dr. James Catto. At time of presentation, Cohort 2 had enrolled 10 patients with a median age of 72 years. The complete response rates at the 1st (Cycle 3, Day 1) and 2nd evaluations (Cycle 6, Day 1) were 100% and 75%, respectively. At a median follow-up of 10 months, the median duration of response was 3.0 months. Grade 3 or worse treatment-related adverse events occurred in 3 patients (30%), and included dry mouth, stomatitis, nail disorder, onychomadesis, acute kidney injury, chronic kidney disease, sepsis, and hypotension. One patient discontinued treatment due to adverse events. There were no treatment-related deaths.
Conclusions
Intravesical chemotherapeutic agents have emerged as promising treatment options for patients with BCG unresponsive NMIBC. Given its ease of administration and wide availability, the intravesical combination of gemcitabine plus docetaxel has emerged as one of the most commonly used treatments in this setting in clinical practice. TAR-200, via a local sustained release of gemcitabine, demonstrates excellent early response rates in SunRISE-1. These agents add to the growing armamentarium in the BCG unresponsive disease space with future regulatory approvals by the FDA contingent on further follow-up of current and future studies of these agents.
Published September 2023
- Written by: Rashid K. Sayyid, MD, MSc University of Toronto Toronto, ON and Zachary Klaassen, MD, MSc Medical College of Georgia Augusta, Georgia, USA
- References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer. https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer. Accessed on July 30, 2023.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Schmidt S, Kunath F, Coles B, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Review. 2020;1(1):CD011935.
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 Update. Eur Urol. 2019;76(5):639–57.
- FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer. Accessed on July 30, 2023.
- FDA D.I.S.C.O. Burst Edition: FDA approval of Adstiladrin (nadofaragene firadenovec-vncg) for patients with high-risk Bacillus Calmette-Guérin unresponsive non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-adstiladrin-nadofaragene-firadenovec-vncg-patients-high-risk#:~:text=On%20December%2016%2C%202022%2C%20the,with%20or%20without%20papillary%20tumors. Access on July 30, 2023.
- Boorjian SA, Alemozaffar M, Konety BR, Shore ND, Gomella LG, Kamat AM, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021;22(1):107–117.
- O’Donnell MA, Lilli K, Leopold C, National Bacillus Calmette-Guerin/interferon phase 2 investigator G Interim results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alfa-2b for superficial bladder cancer. J Urol. 2004;172(3):888–93.
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-Institution Evaluation of Sequential Gemcitabine and Docetaxel as Rescue Therapy for Nonmuscle Invasive Bladder Cancer. J Urol. 2020;203(5):902-9.
- Chevuru PT, McElree IM, Mott SL, et al. Long-term follow-up of sequential intravesical gemcitabine and docetaxel salvage therapy for non-muscle invasive bladder cancer. Urol Oncol. 2023;41(3):148.e1-7.
- Daneshmand S, van der Heijden MS, Jacob JM, et al. LBA02-03 FIRST RESULTS FROM SunRISE-1 IN PATIENTS WITH BCG UNRESPONSIVE HIGH-RISK NON–MUSCLE-INVASIVE BLADDER CANCER RECEIVING TAR-200 IN COMBINATION WITH CETRELIMAB, TAR-200, OR CETRELIMAB ALONE. J Urol. 2023;209(Suppl 4):e1187.
- Witjes JA, Hendricksen K, Gofrit O, Risi O, Nativ O. Intravesical hyperthermia and mitomycin-C for carcinoma in situ of the urinary bladder: experience of the European Synergo® working party. World J Urol. 2009;27:319-24.
- Van Valenberg FJP, Kajtazovic A, Canepa G, et al. Intravesical Radiofrequency-Induced Chemohyperthermia for Carcinoma in Situ of the Urinary Bladder: A Retrospective Multicentre Study. Bladder Cancer. 2018;4(4):365-76.
- De Jong JJ, Hendricksen K, Rosier M, Mostafid H, Boormans JL. Hyperthermic Intravesical Chemotherapy for BCG Unresponsive Non-Muscle Invasive Bladder Cancer Patients. Bladder Cancer. 2018;4(4):395-401.
- Tan WP, Plata Bello A, Garcia Alvarez C, et al. A Multicenter Study of 2-year Outcomes Following Hyperthermia Therapy with Mitomycin C in Treating Non-Muscle Invasive Bladder Cancer: HIVEC-E. Bladder Cancer. 2022;8(4):379-93.
- Alzeibak R, Mishchenko TA, Shilyagina NY, et al. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021;9:e001926.
- Meng Z, Zhou X, Xu J, et al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv Mater. 2019;31:e1900927.
- Kulkarni GS, Richards KA, Black PC, et al. A phase II clinical study of intravesical photo dynamic therapy in patients with BCG-unresponsive NMIBC (interim analysis). J Clin Oncol. 2023;6(Suppl):528.
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021;384:1125-35.
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019;381:338-48.


