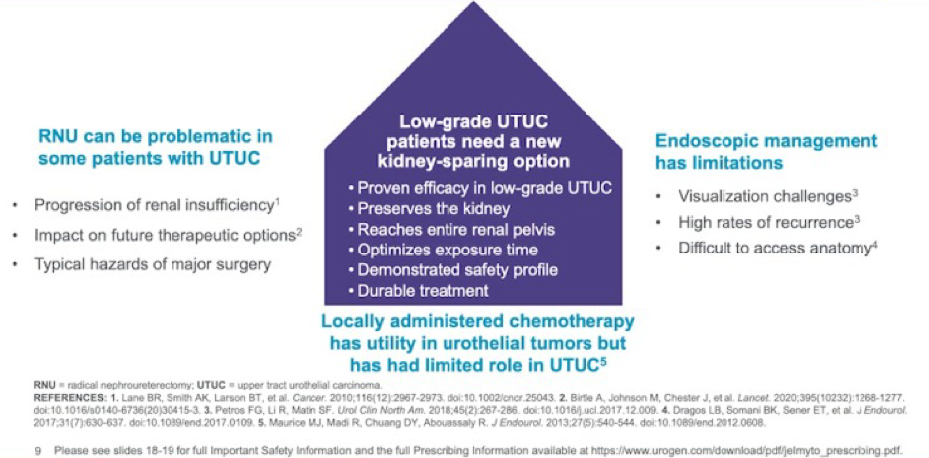
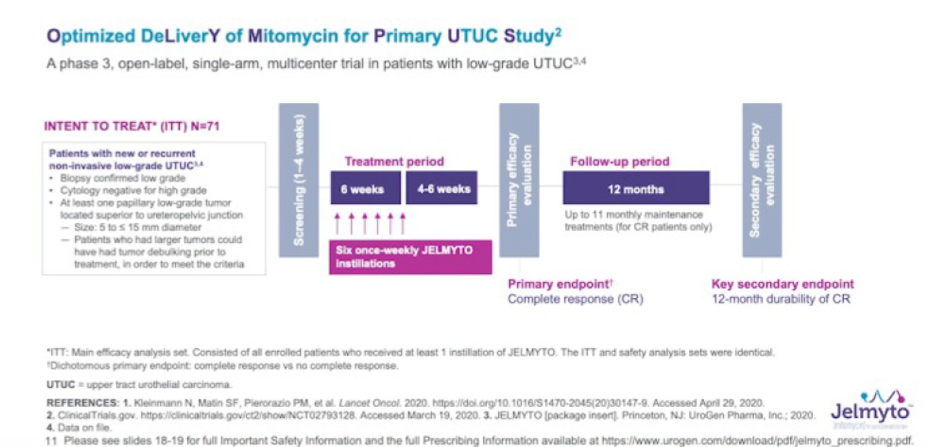
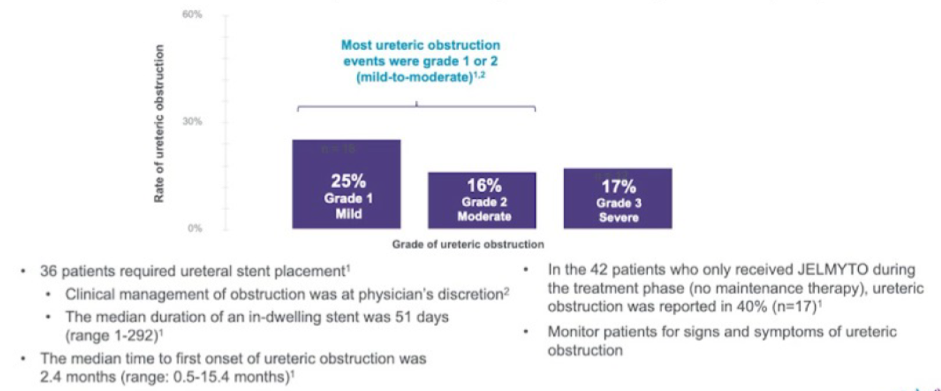
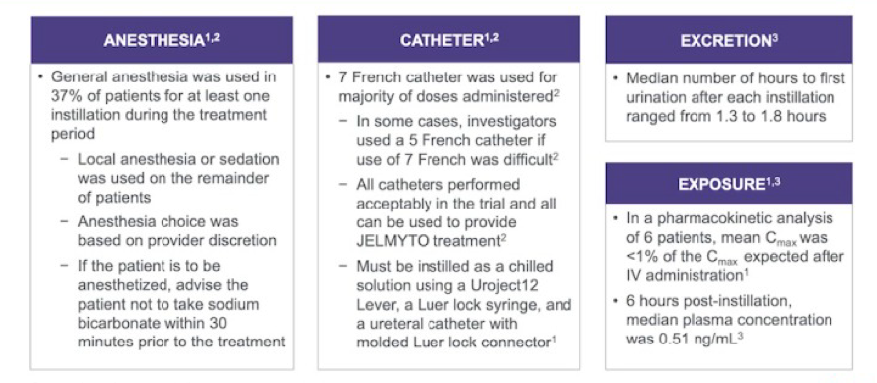
AUA 2020: Identifying the Frequency of Actionable Genomic Alterations in Localized and Metastatic Upper Tract Urothelial Carcinoma
(UroToday.com) Upper tract urothelial carcinoma tumors have distinct genomic profiles that distinctly differentiate them from bladder cancer tumors.
In the presented study, the authors characterized the prevalence of actionable genomic alterations and evidence supporting susceptibility to targeted therapies. In addition, they evaluated the prevalence of DNA damage response alterations associated with response to platinum-based chemotherapy and PD-1/PD-L1 blockade.
It has been shown that key differences in the genomic landscape of high-grade upper tract urothelial carcinoma can have the potential to alter treatment paradigms.
For this study, upper tract urothelial carcinoma tumors that underwent next-generation sequencing using the MSK-IMPACT platform were identified from a prospectively maintained institutional database.
Patients were grouped by clinical disease state (localized vs. metastatic) at the time of sequencing. Actionable cancer gene alterations were identified using the OncoKB platform and classified based on the level of evidence. Additionally, the authors identified 34 genes within the MSK-IMPACT as DDR-related based on Pubmed searches and NCBI gene and biosystems database.
In 2019, Erdafitinib received FDA approval for the treatment of urothelial tumors, which harbored fusions in FGFR2 or FGFR3 and oncogenic mutations in FGFR3.1
Alterations resulting in loss of function in mismatch repair genes can lead to microsatellite instability-high phenotype. Mismatch repair status has been shown to be predictive of clinical benefit from pembrolizumab across all solid tumor carcinomas.2
In this cohort, a total of 206 tumors were sequenced (145 localized, and 61 metastatic). At least one actionable alteration was found in 91% of the cohort (93% of patients with localized disease, and 89% of patients with metastatic disease), as seen in Figure 1.
Figure 1 – Genomic alterations identified in the cohort:

Somatic alterations in genes associated with DNA damage response and repair are associated with enhanced platinum responsiveness, leading to a higher likelihood of pathological downstaging in neoadjuvant treated bladder cancers(3). Data from the authors’ institution also suggest that DDR mutations are associated with improved anti-PD1/PD-L1 responsiveness, possibly through modulation of tumor mutational burden(4).
At least one DDT alteration was found in 39% of the overall UTUC cohort (38% of clinically localized and 40% of metastatic patients), as seen in Figure 2.
Figure 2 – Frequency of alteration in DNA damage repair genes:

The authors concluded that most tumors evaluated in this study harbor actionable genomic alterations in both the localized and the metastatic setting, demonstrating the important role genetic testing may play in the management of UTUC.
These presented findings could hopefully be used to guide rational in planning clinical trial design with targeted therapies.
Presented by: Andrew Tracey, MD, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27-28, 2020.
References:
- Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. New England Journal of Medicine. 2019;381(4):338-48.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New England Journal of Medicine. 2015;372(26):2509-20.
- Teo MY, Bambury RM, Zabor EC, Jordan E, Al-Ahmadie H, Boyd ME, et al. DNA Damage Response and Repair Gene Alterations Are Associated with Improved Survival in Patients with Platinum-Treated Advanced Urothelial Carcinoma. Clinical Cancer Research. 2017;23(14):3610-8.
- Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. Journal of Clinical Oncology. 2018;36(17):1685-94.