(UroToday.com) The 2024 ASTRO annual meeting included a late-breaking abstract session, featuring a presentation by Dr. Ana Kiess discussing outcomes of the RAVENS phase 2 randomized trial assessing outcomes of radium-223 + stereotactic ablative radiotherapy versus stereotactic ablative radiotherapy alone for oligometastatic prostate cancer. Previously, the STOMP1 and ORIOLE2 randomized clinical trials showed progression-free survival benefits of metastasis-directed therapy alone without ADT for oligometastatic hormone-sensitive prostate cancer:
However, most patients (~86%) with bone metastatic oligometastatic hormone-sensitive prostate cancer recur with additional bone metastases following metastasis-directed therapy alone:
Dr. Kiess and colleagues hypothesized that the addition of bone metastatic-targeting alpha-emitter radium-223, approved for treatment of bone metastases castration-resistant prostate cancer (mCRPC)3 showing an improvement in overall survival for radium-223 versus placebo (14.9 versus 11.3 months), could delay progression of disease. Moreover, at ESMO 2024, Silke Gillessen presented data from PEACE-3 showing that radium-223 in combination with enzalutamide for first-line mCRPC improved overall survival over enzalutamide alone (HR 0.69, 95% CI 0.52-0.90). In addition, biomarkers to determine patients who benefit most from metastasis-directed therapy are still poorly defined. At ASTRO 2024, Dr. Kiess reported on the first randomized clinical trial to examine radium-223 and evaluate novel biomarkers in bone metastatic oligometastatic hormone-sensitive prostate cancer.
In this phase 2 multi-institutional randomized clinical trial (NCT04037358), men with recurrent oligometastatic hormone-sensitive prostate cancer (>1 bone metastases & <5 radiation fields) were stratified by institution, primary management (radiotherapy vs surgery), PSA doubling time and prior ADT, and subsequently randomized 1:1 to stereotactic ablative radiation metastasis-directed therapy or stereotactic ablative radiation and 6 monthly cycles of radium-223. The trial schema for RAVENS is as follows:

The primary endpoint was composite progression-free survival:
- >= 25% PSA increase and >= nadir + 2 ng/mL and/or
- Radiological progression by RECIST 1.1 or new lesion on bone scan and/or
- Symptomatic progression (worsening disease-related symptoms or new cancer-related complications) and/or
- ADT initiation or death due to any cause
Secondary endpoints included:
- ADT free survival
- Metastasis free survival
- Overall survival
- Patterns of progression
- Toxicity and quality of life
- Molecular studies: germline and somatic DNA sequencing, circulating tumor cells, T-cell receptor sequencing, proteomics, and ctDNA sequencing using CAPP-Seq
Tissue, liquid, and imaging correlative studies were obtained and analyzed as biomarkers.
From August 2019 – March 2023, 64 patients were randomized: 33 to stereotactic ablative radiation and 31 to stereotactic ablative radiation/radium-223. The arms were balanced for key covariates and 26 (87%) patients received the full 6 planned cycles of radium-223:
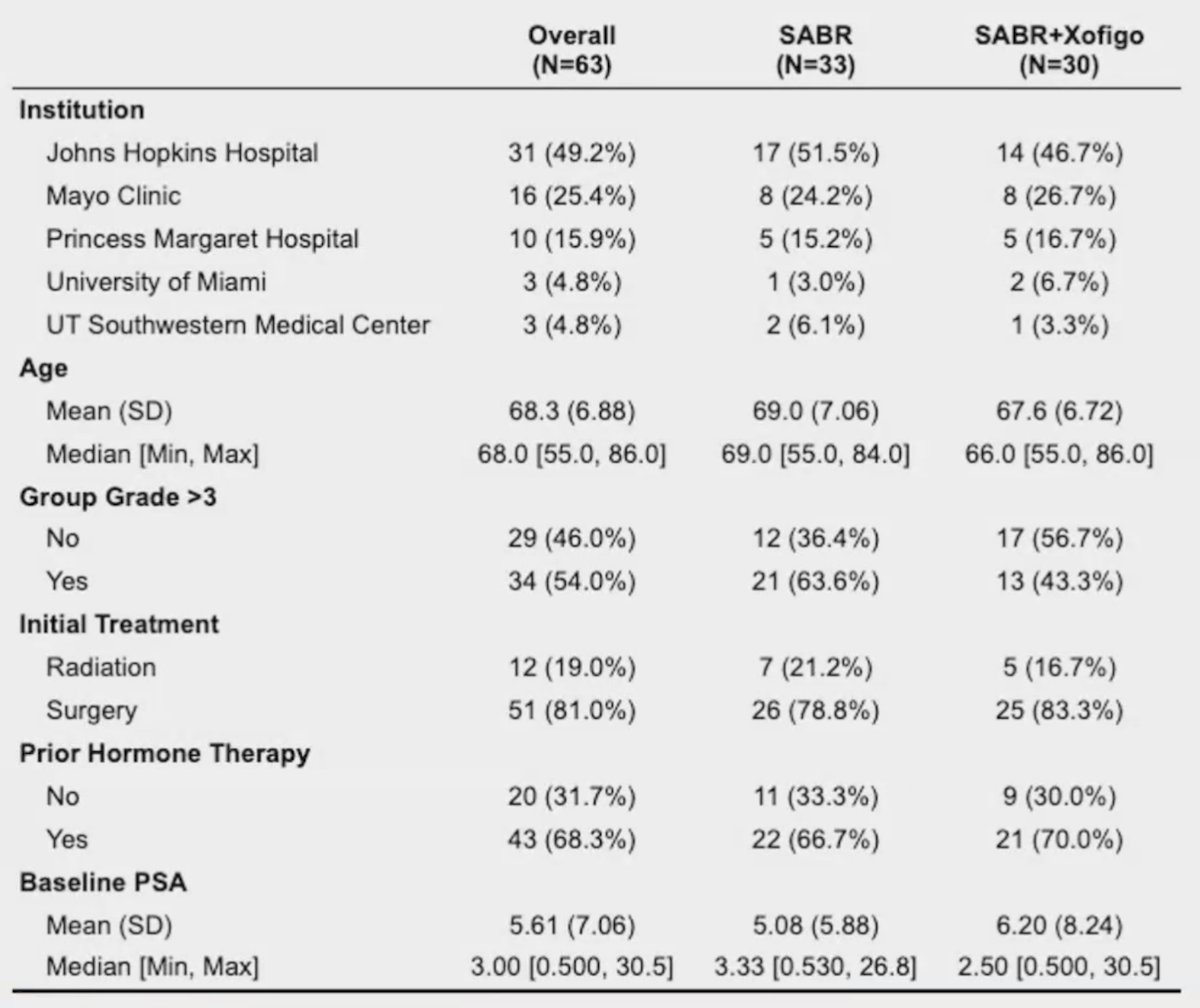
Over a median follow-up of 18.7 months, the median progression-free survival was 11.8 months with stereotactic ablative radiation and 10.5 months with stereotactic ablative radiation/radium-223 (stratified HR 1.37, 95% CI, 0.78-2.39, p = 0.27):
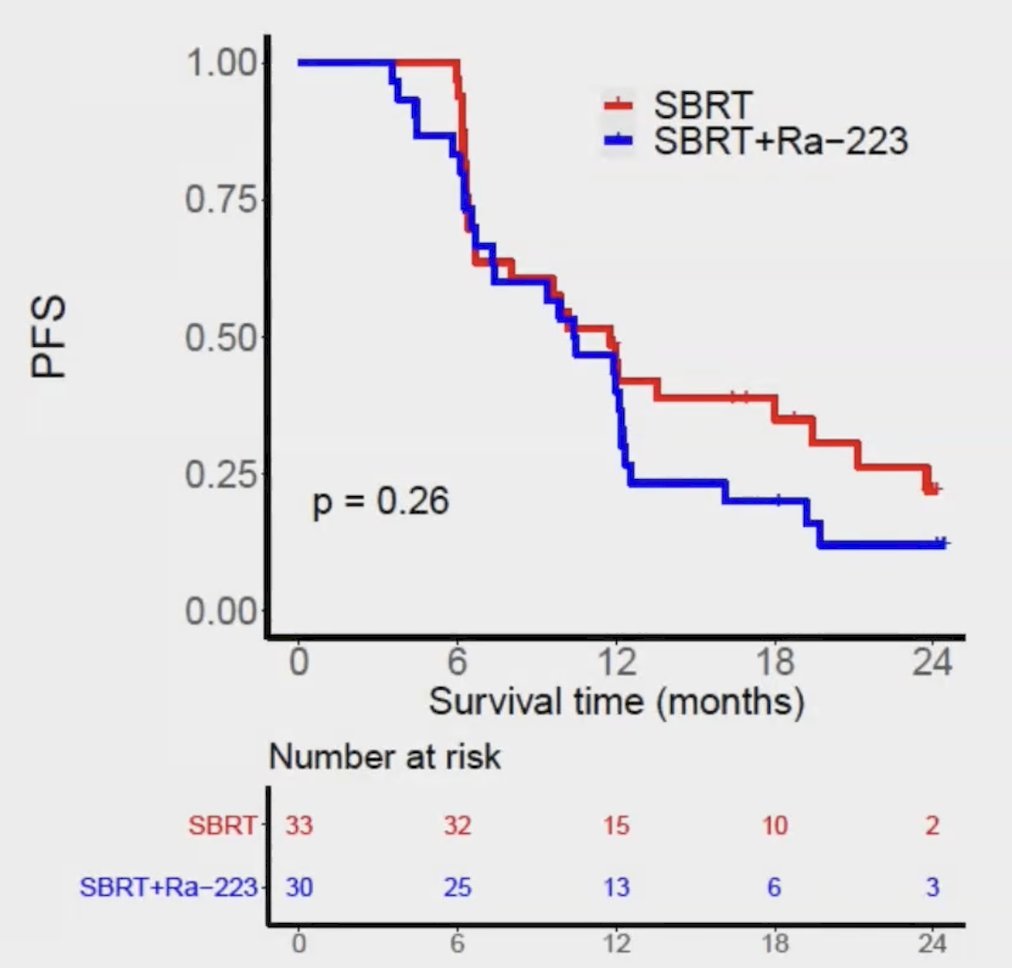
Additionally, there was no difference between arms with regard to metastasis-free survival and ADT-free survival:
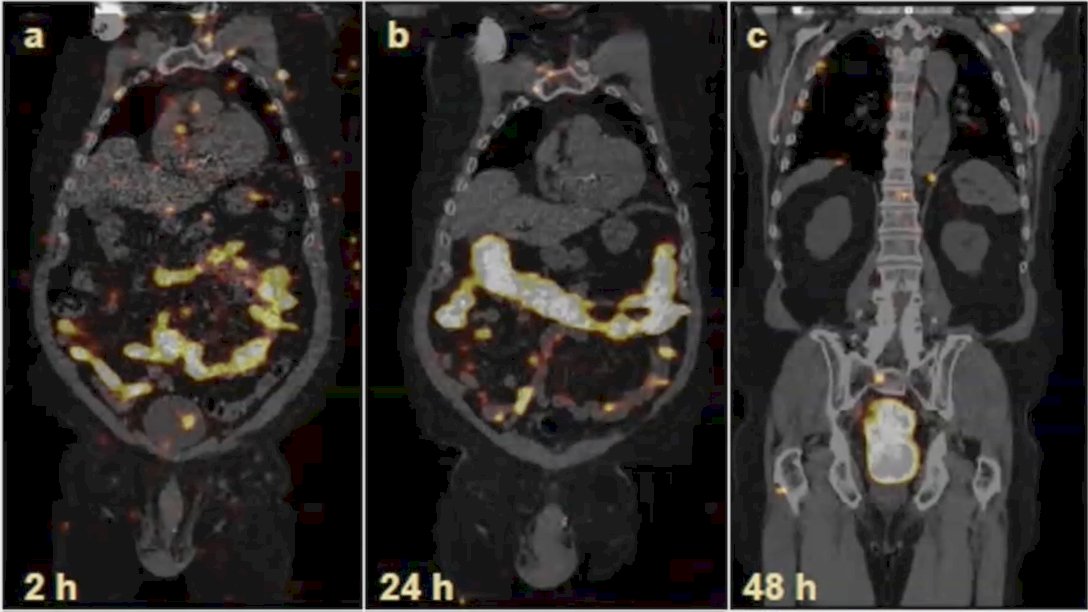
Dr. Keiss notes that perhaps one of the reasons radium-223 did not work in oligometastatic disease is due to the mechanism of action in that radium-223 is likely most effective in higher volume disease with bone lesions not eradicated by stereotactic ablative radiotherapy. In a dosimetry/treatment optimization sub-study of RAVENS, SPECT imaging demonstrated tumor uptake based on 5 patients imaged at 2, 24, and 48 hours after cycle 1 and cycle 6:
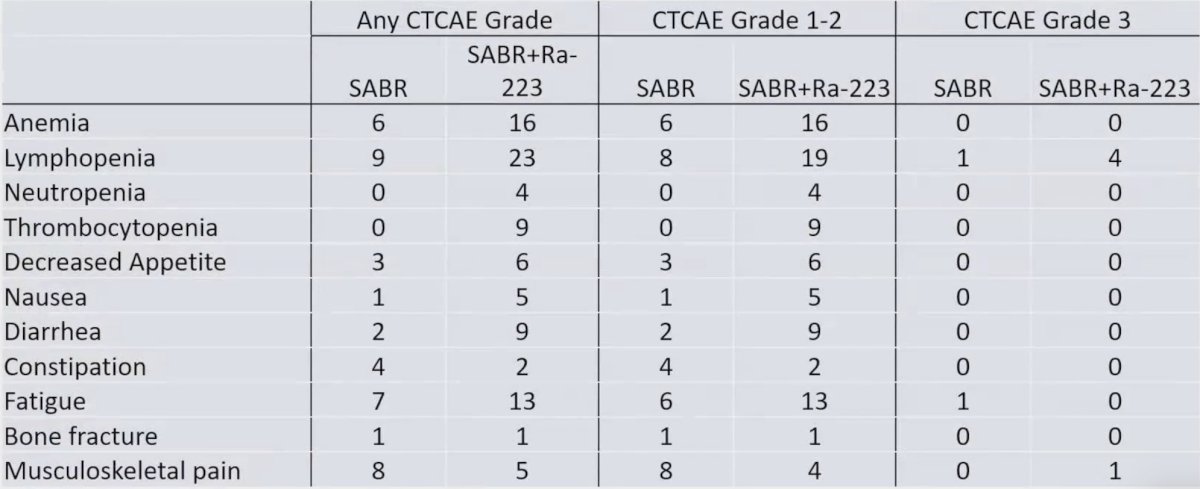
Overall, 7 patients experienced grade 3 toxicity (no grade 4 or 5), of which lymphopenia was the most common. There were more grade 1-2 hematologic and gastrointestinal toxicities related to radium-223 + stereotactic ablative radiotherapy:
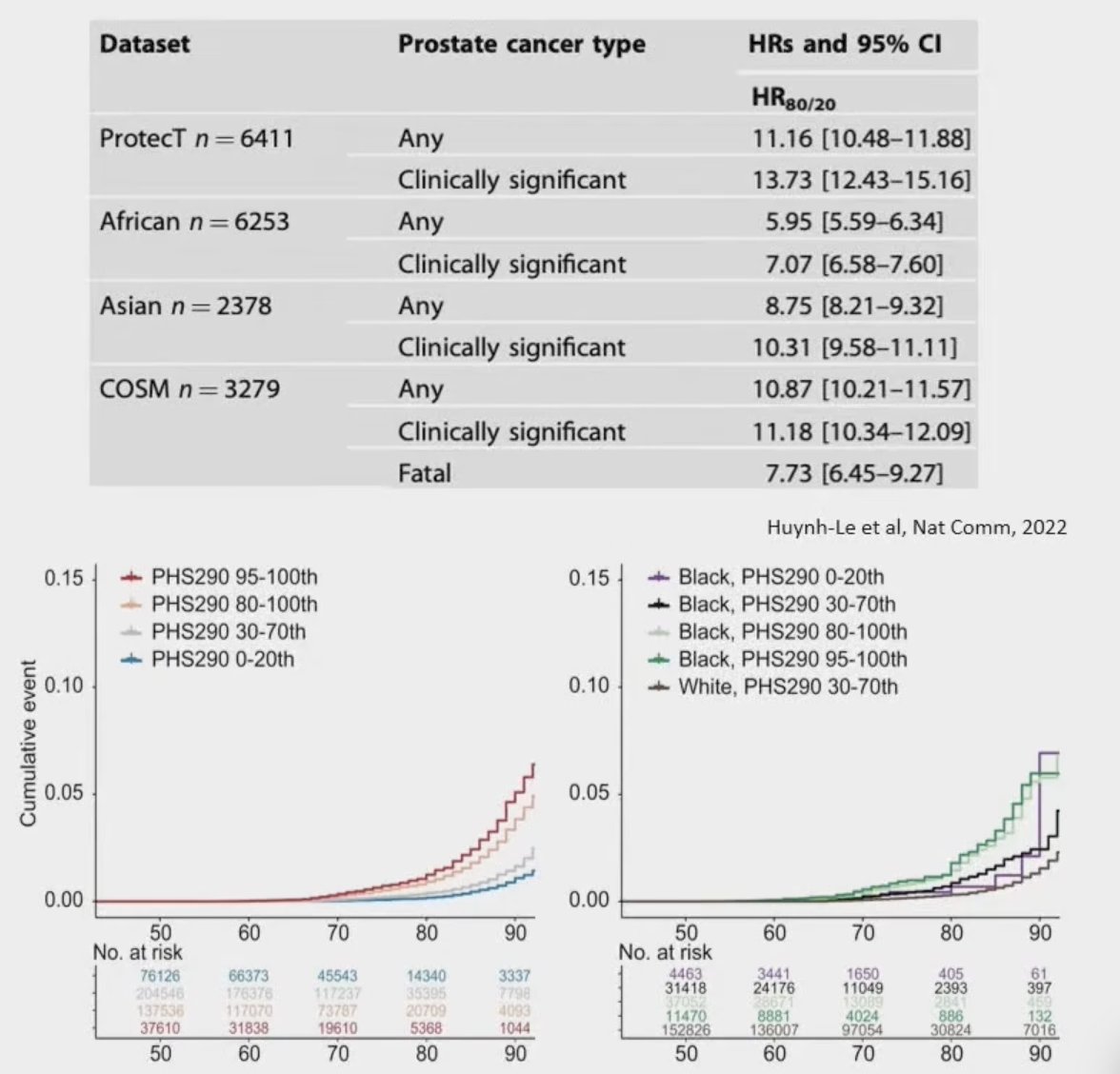
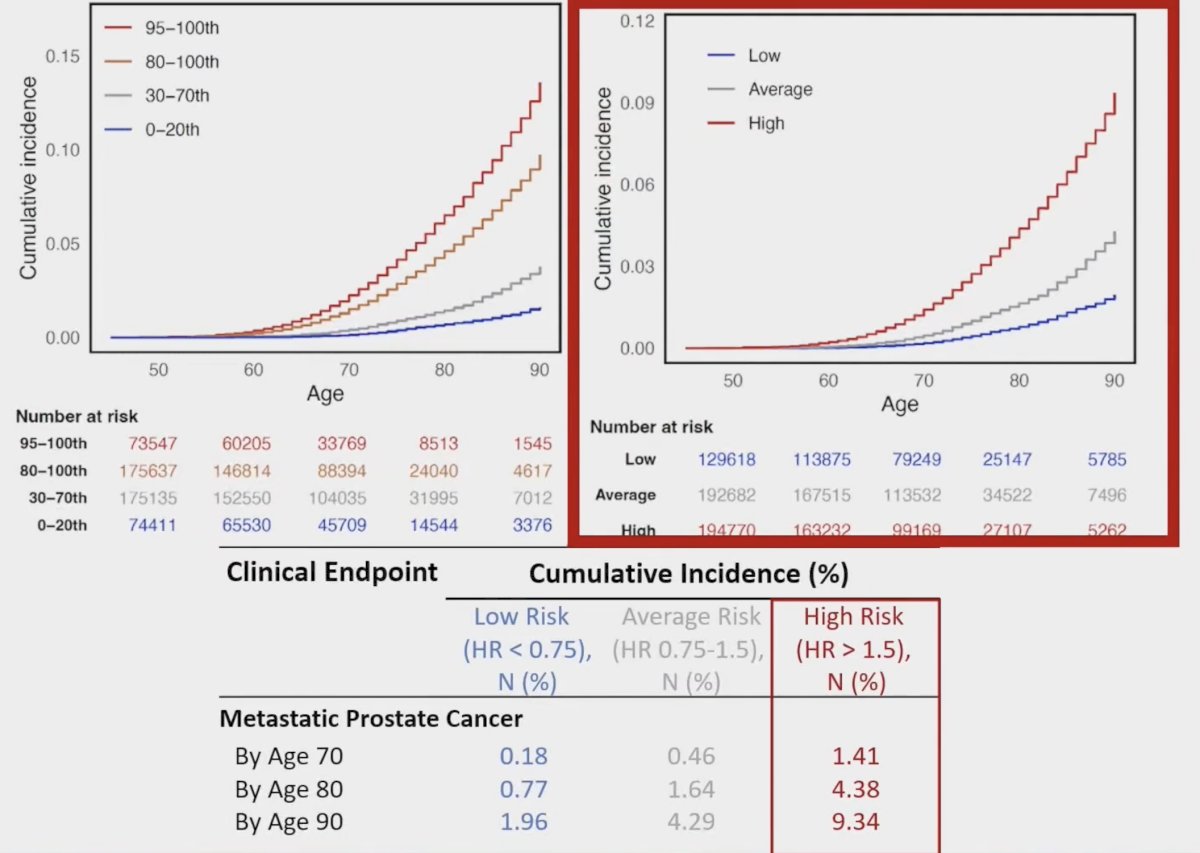
A high-risk genomic signature, noting pathogenic mutations in ATM, BRCA1, BRCA2, RB1, or TP53 was previously assessed in STOMP and ORIOLE, showing the largest benefit of metastasis-directed therapy in patients with high-risk mutations. In RAVENS, this high-risk genomic signature was validated in 23 patients, which was prognostic for progression-free survival (median 12.5 months for low-risk versus 6.5 months for high-risk); HR 5.95, 95% CI 1.83-19.3:

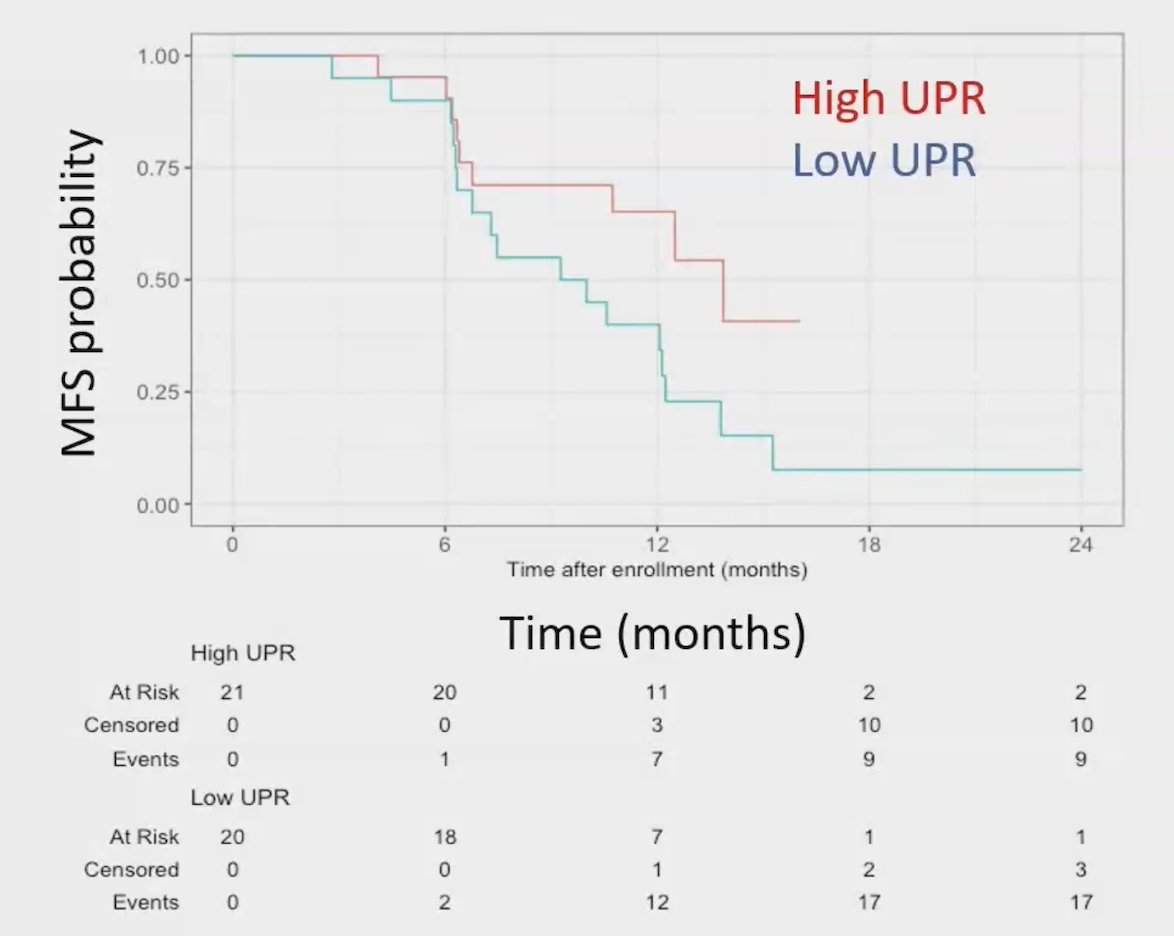
T-cell receptor samples were available at baseline (44/63, 70%), at 90 days of follow-up (42/63, 67%), and with paired samples (37/63, 59%). Greater unique productive T-cell receptor rearrangements were prognostic for progression-free survival independent of treatment arm (adjusted HR 0.45, 95% CI, 0.21-0.96; p = 0.04):
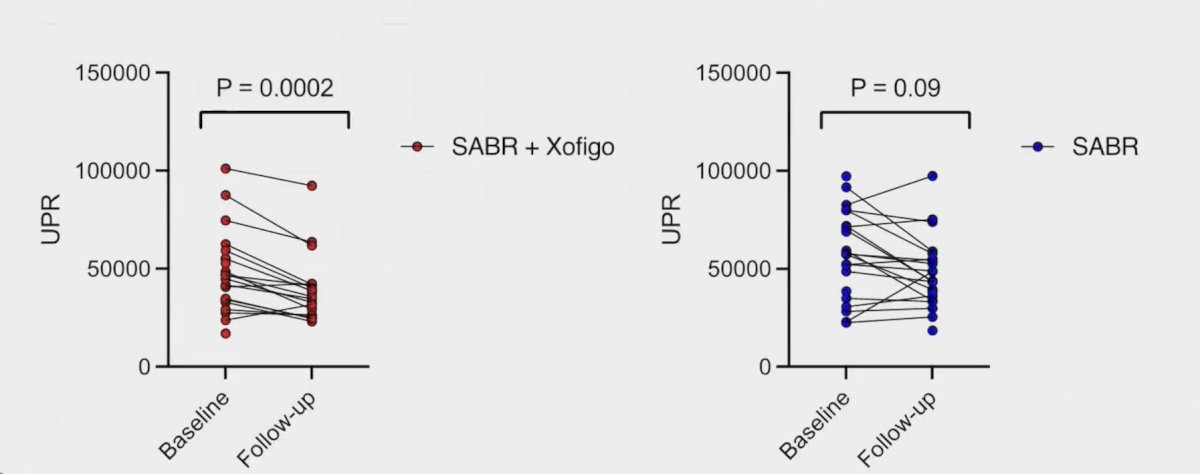
Additionally, unique productive T-cell receptor rearrangements were reduced with radium-223 + stereotactic ablative radiotherapy, but not in stereotactic ablative radiotherapy alone:
Finally, Dr. Keiss showed that T-cell receptor clonal expansion still occurs after stereotactic ablative radiotherapy with or without radium-223:
Dr. Keiss concluded her presentation discussing outcomes of the RAVENS phase 2 randomized trial with the following take-home points:
- Stereotactic ablative radiotherapy alone for oligometastatic hormone-sensitive prostate cancer affords progression-free survival benefits, but emergence of additional bone metastases in most patients remains a challenge
- This is the first report noting that the addition of radium-223 to stereotactic ablative radiotherapy metastasis-directed therapy in this low-volume bone metastatic state does not delay progression of disease
- This trial validates the T-cell receptor repertoire as a prognostic biomarker in oligometastatic hormone-sensitive prostate cancer treated with stereotactic ablative radiotherapy metastasis-directed therapy
- These results underline the importance of randomized clinical trials in oligometastatic hormone-sensitive prostate cancer with concurrent collection of biological correlates
Presented by: Ana Kiess, MD, PhD, Radiation Oncologist, Johns Hopkins University, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting, Washington, DC, Sun, Sept 29 – Wed, Oct 2, 2024.
References:
- Ost P, Reynders D, Decaestecker K, et al. Surveillance of metastasis-directed therapy for oligometastatic cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018 Feb 10;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020 Mar 26;6(5):650-659.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.