ASCO GU 2024
ASCO GU 2024
ASCO GU 2024: Update on Biomarkers in Renal Cell Carcinoma
(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a session addressing the incorporation of radiation therapy, theranostics, and biomarkers into the management of renal cell carcinoma (RCC). Dr. David Braun provided an update on biomarkers in RCC.
ASCO GU 2024: Biology of Tumor Types: Radiation Versus Surgery
(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a session on optimizing treatment strategies for patients with positive pelvic and/or retroperitoneal lymph nodes.
ASCO GU 2024: Phase 3 Open-Label, Randomized, Controlled Study of Disitamab Vedotin with Pembrolizumab Versus Chemotherapy in Patients with Previously Untreated Locally Advanced or Metastatic Urothelial Carcinoma That Expresses HER2 (DV-001)
(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma trials in progress poster session.
ASCO GU 2024: Catalysts for Change: Patient Advocacy’s Role in Drug Shortages
(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a session addressing the shortage of drugs for urothelial carcinoma patients and, specifically, why practitioners struggle to get patients what they need and what can we do about this.
ASCO GU 2024: Approach to the Platinum Shortage from a Community Oncologist Perspective
(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a session addressing the shortage of drugs for urothelial carcinoma patients and, specifically, why practitioners struggle to get patients what they need and what can we do about this. Dr. Elizabeth Guancial discussed potential approaches to the ongoing platinum shortage from a community oncologist’s perspective.
ASCO GU 2024: Phase 3 KEYNOTE-676 Cohort A: Bacillus Calmette-Guérin with or Without Pembrolizumab for High-Risk Non–muscle-Invasive Bladder Cancer That Persists/recurs After BCG Induction
(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma trials in progress poster session. Dr. Neal Shore presented the study design and provided an update for the phase 3 KEYNOTE-676 Cohort A trial of BCG +/- pembrolizumab for patients with high-risk non-muscle invasive bladder cancer (HR NMIBC) that persists/recurs following BCG induction.
ASCO GU 2024: Advanced Urothelial Carcinoma Discussant
The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma oral abstract session. Dr. Parminder Singh delivered the discussant session for the two previously presented abstracts:
- Enfortumab vedotin (EV) in combination with pembrolizumab versus chemotherapy in previously untreated locally advanced/metastatic urothelial carcinoma: Subgroup analyses results from EV-302, a phase 3 global study
- Cabozantinib plus pembrolizumab as first-line therapy for cisplatin-ineligible advanced urothelial carcinoma (PemCab)
PemCab is a phase II trial that evaluated the activity and safety of 1st line combination of cabozantinib and pembrolizumab in (cisplatin-ineligible/PD-L1 positive) and cisplatin-refusing or platinum ineligible locally advanced/metastatic urothelial carcinoma. The PemCab study design is illustrated below. Eligible patients were administered pembrolizumab 200 mg IV every 3 weeks plus cabozantinib 40 mg orally once daily. The primary endpoint was objective response rate (ORR), per RECIST v1.1.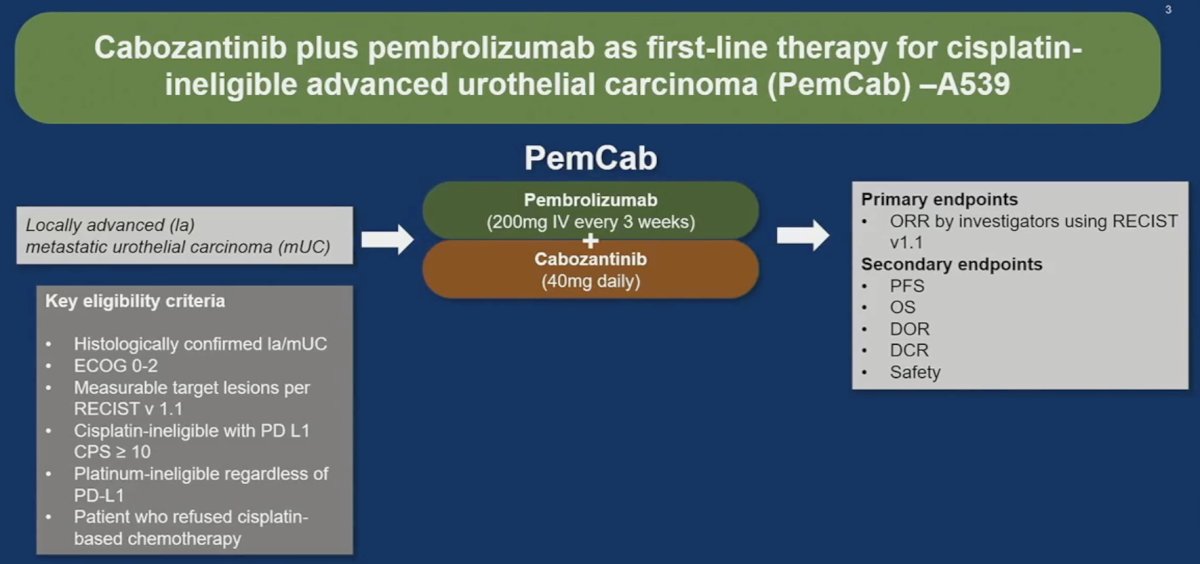
This trial enrolled 36 patients. An ORR was observed in 16 patients (46%), of whom 4 (14%) had a complete response. On radiographic evaluation, 80% of patients demonstrated evidence of tumor shrinkage. While notable, these were not ‘groundbreaking’ results, per Dr. Singh. Furthermore, many patients experienced fatigue (64%), diarrhea (58%), pruritis (39%), and anorexia (33%), among other side effects. As such, he argued that the risk-profile for such a combination is unclear. He noted that this trial adds to the litany of negative TKI/IO or VEGF/chemotherapy phase 2/3 trials in urothelial carcinoma. As such, should this spell the end for such combinations in urothelial carcinoma, or should they be explored in alternate settings?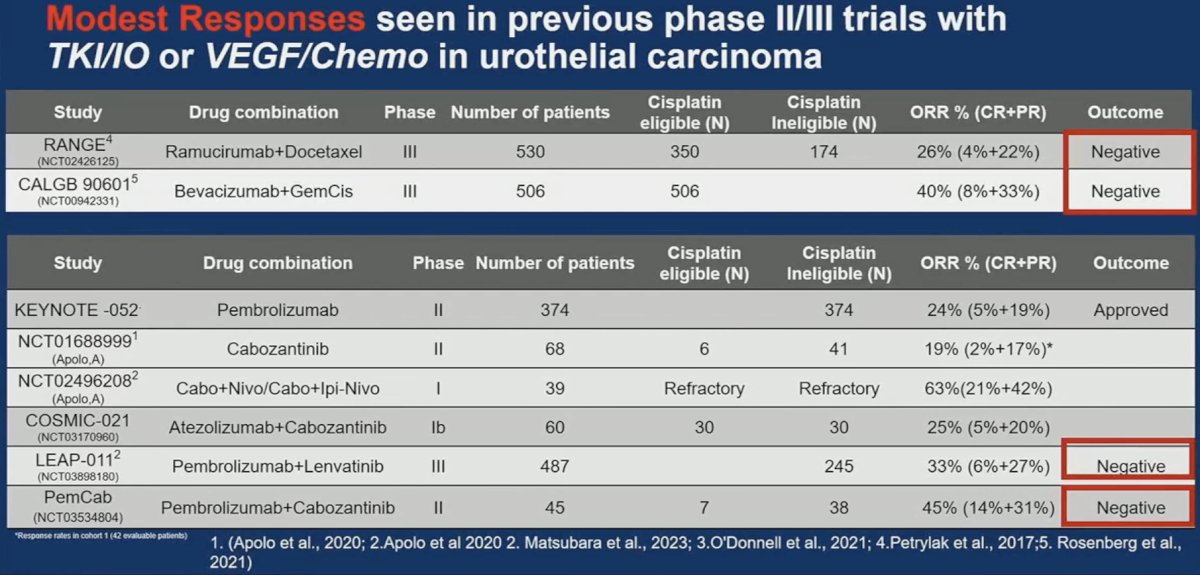
Based on these results, Dr. Singh concluded that the PemCab combination shows modest activity as 1st line novel combination. Given the evolving paradigm of 1st line therapy, it is unlikely that this combination will have a future role in 1st line therapy for advanced urothelial carcinoma.
With regards to potential future directions in this space and for such combinations, new multi-kinase inhibitors like XL092 (similar to cabozantinib) with better tolerability/therapeutic indices could be used:
- In combination with pembrolizumab in the maintenance setting in patients who are responding/stable to enfortumab vedotin + pembrolizumab (to avoid cumulative neuropathy from enfortumab vedotin)
- Cabozantinib + nivolumab could reinvigorate immune responses in IO-refractory patients. Newer multi-kinase inhibitors can similarly be explored.
- May be investigated as salvage monotherapy, potentially informed by predictive biomarkers (lack of neurotoxicity could be advantageous)
Next, Dr. Singh discussed the subgroup analyses of EV-302 presented by Dr. van der Heijden. He noted that cisplatin eligibility has long defined treatment in this disease space. The seminal paper by Dr. Galsky in 2011 has long defined cisplatin eligibility for these patients and has shaped clinical trial design since.1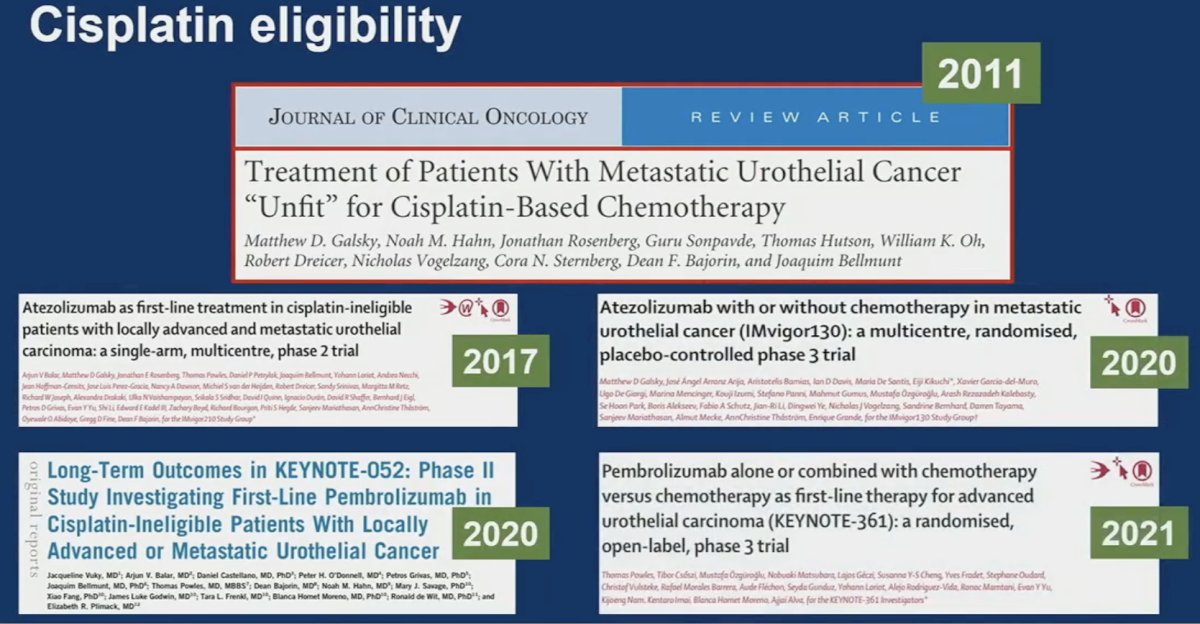
This had shaped the pre-EV-302 treatment paradigm of advanced urothelial carcinoma. Cisplatin eligible patients would receive gem/cis or ddMVAC, those who were cisplatin ineligible would receive enfortumab vedotin, Gem/Carbo, or Gem/Paclitaxel, and those who were chemo ineligible would receive pembrolizumab.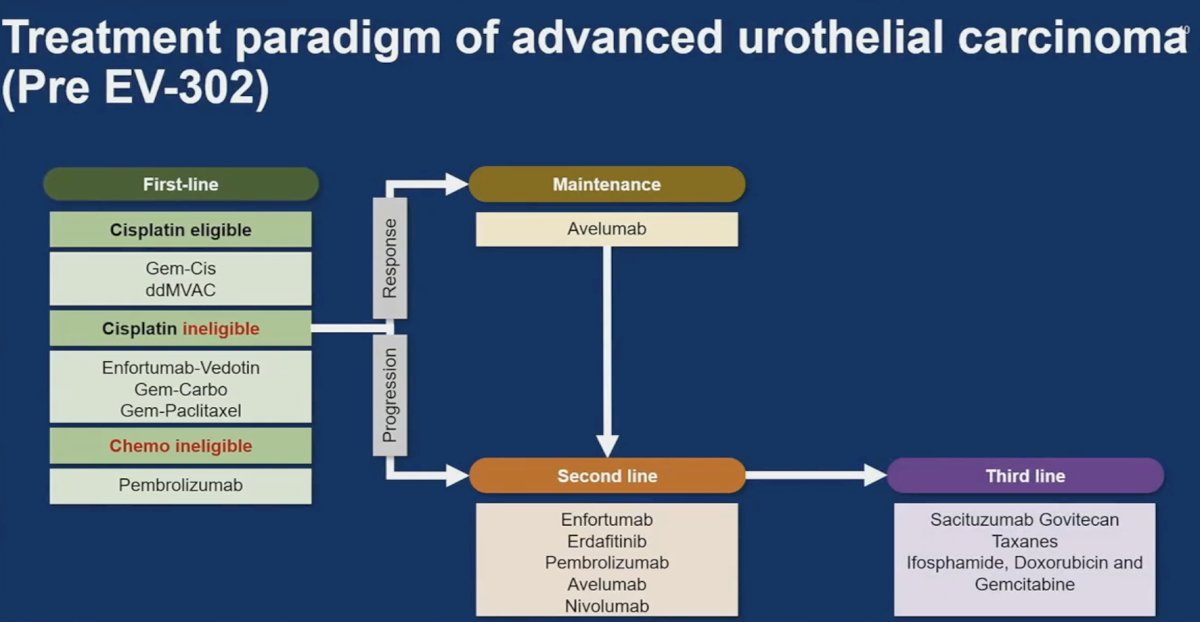
What was its impact in the clinic? Real world data of patients from the Flatiron database between 2016 and 2020 demonstrated that ~25% of patients did not receive 1st line therapy, and half of patients did not receive 2nd line therapy. Among those who were cisplatin eligible, only 40% received cisplatin combination chemotherapy, and 25% received immunotherapy. Conversely, among those who were cisplatin ineligible, 50% received immunotherapy.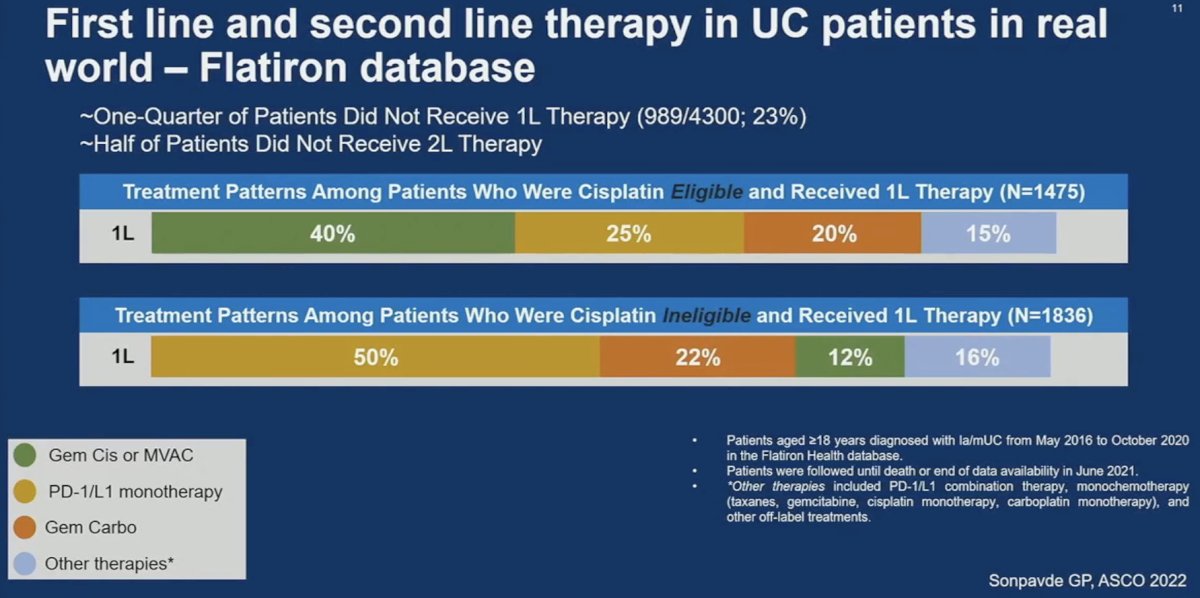
He noted that EV-302 challenges this paradigm. EV-302 is a phase 3 global trial of enfortumab vedotin in combination with pembrolizumab versus chemotherapy in previously untreated locally advanced metastatic urothelial carcinoma. There was no maximum number of treatment cycles for enfortumab vedotin and up to 35 cycles of pembrolizumab were allowed. All included patients were platinum and immunotherapy eligible.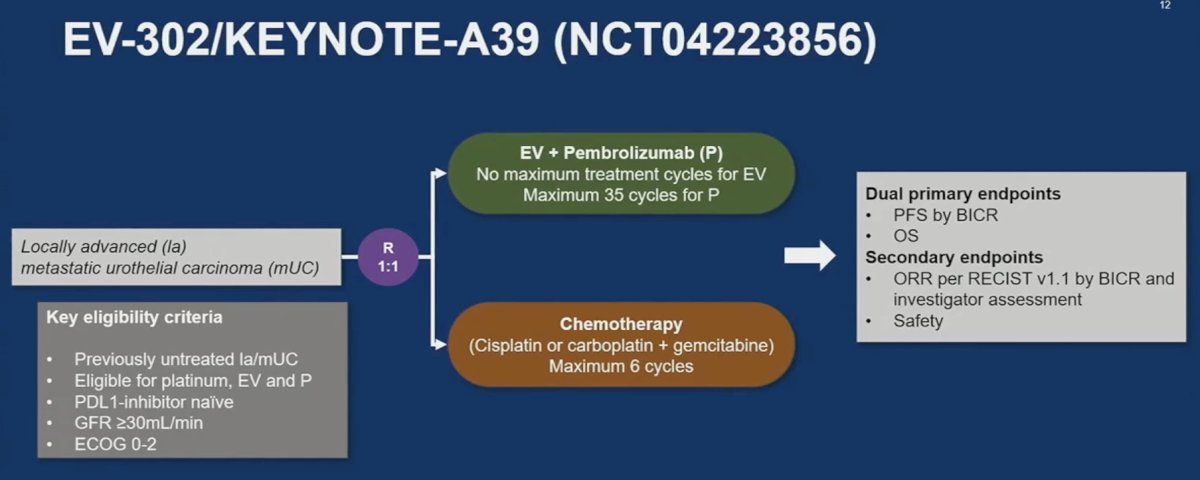
The objective response rates were ≥60% for enfortumab vedotin + pembrolizumab across all evaluable subgroups.
He noted that these ORR results correlate strongly with those observed in the earlier, smaller phase studies of this combination: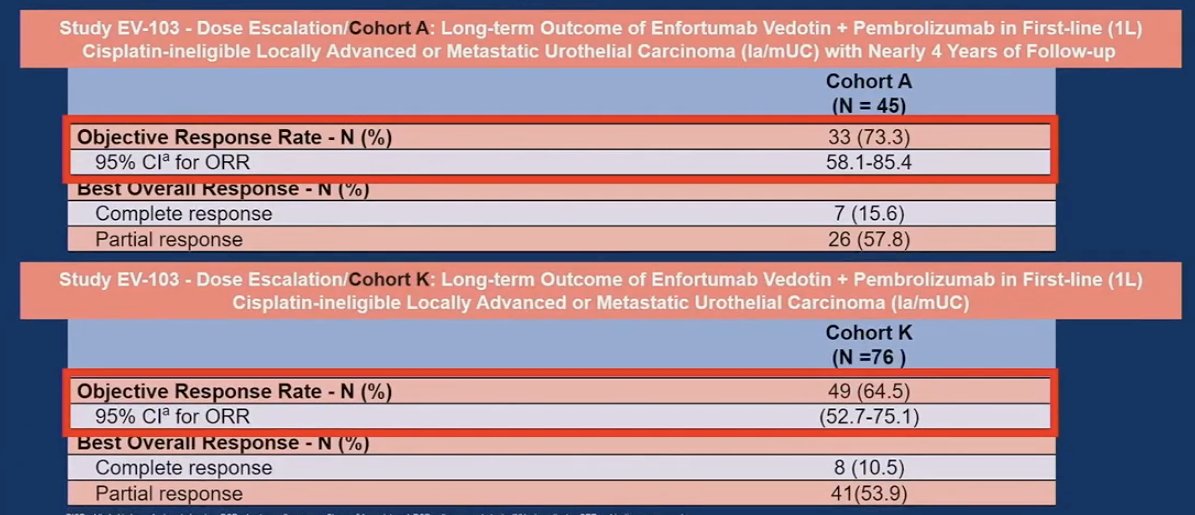
He noted that with the impressive results of EV-302, the combination of enfortumab vedotin + pembrolizumab is likely to be used much more commonly in the 1st line setting. As such, we need to be familiar with the safety/adverse event profile of this combination and learn how to manage them, including skin reactions, peripheral neuropathy, ocular disorders, and hyperglycemia.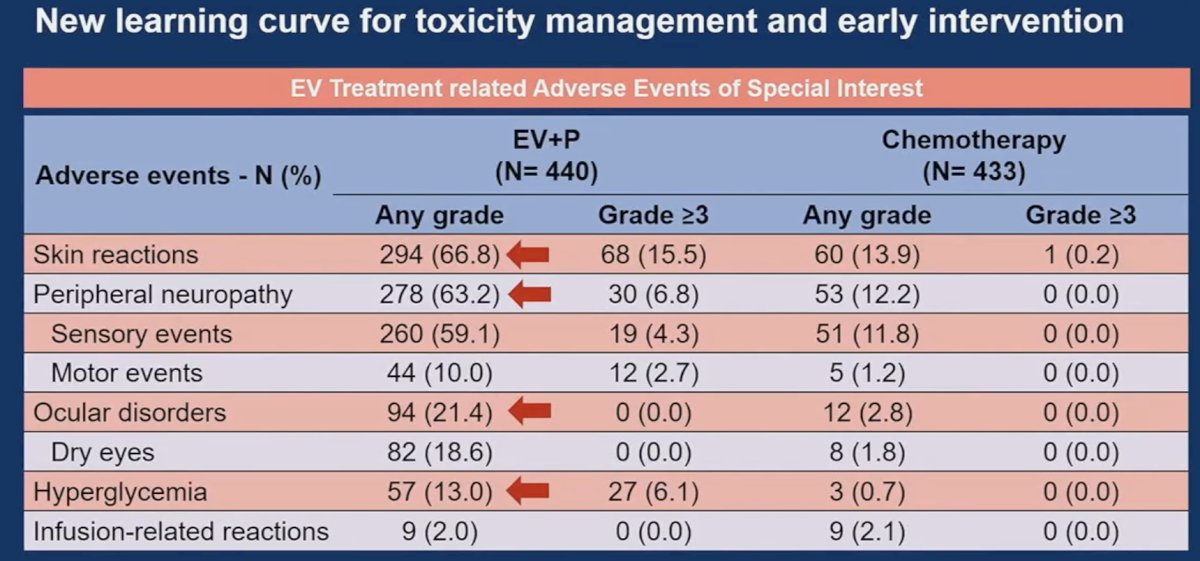
In light of these ‘novel’ side effects, he suggested the following toxicity management ‘pearls’:
- Early dose reduction of enfortumab vedotin for neuropathy – 1 mg/kg or 0.75 mg/kg
- Growth factor support for cytopenia
- Topical steroids and dose reduction for skin rash - use lotion, not ointment
- Strict diabetes management
- Ocular toxicity
- And treatment interruptions in exceptional responders - need to be investigated
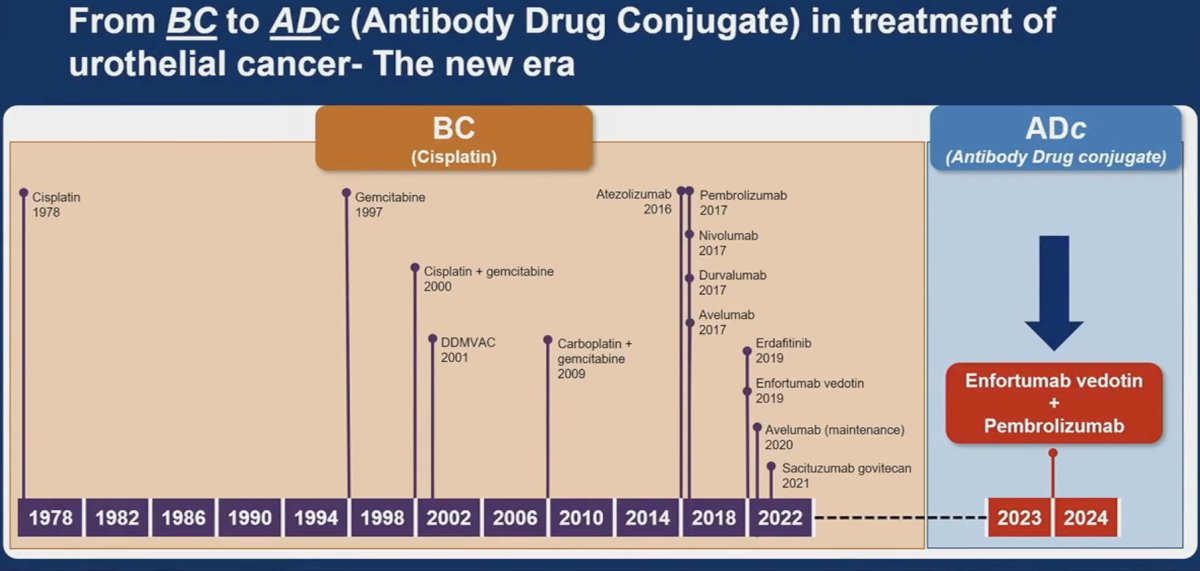
Dr. Singh noted that EV-302 has made it ‘simple for the community oncologist’, whereby this combination is safe for advanced urothelial carcinoma patients meeting the following criteria:
- Cisplatin eligibility (safe up to 30 ml/min GFR)
- ECOG <2
- High-risk group like liver metastases 0 median time to response in the 1st two months
- No risk of cardiac overload
- No need to think about PD-L1 score
- No need to wait for next generation sequencing or any specialized testing
His concluding takeaways from the EV-302 trial were as follows:
- Enfortumab vedotin + pembrolizumab is the new standard of care for locally advanced/metastatic urothelial carcinoma patients.
- There were consistent benefits across various subgroups including cisplatin eligible/ineligible, visceral metastasis (present/absent), and irrespective of PD-L1 status
- It is unclear if enfortumab vedotin + pembrolizumab is curative for some patients.
- Need data on durability of CRs and longer follow-up
- Gemcitabine/Cisplatin + nivolumab (awaiting FDA review) may play a role in selected cisplatin-eligible patients who are predicted to have a CR
- The JAVELIN paradigm and pembrolizumab monotherapy may continue to play a role in select frail or poor performance status patients with significant comorbidities
Presented by: Parminder Singh, MD, Assistant Professor of Hematology and Oncology, Mayo Clinic, Phoenix, AZ
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Reference:- Galsky MD, et al. Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):2432-8.
ASCO GU 2024: Keynote Lecture - Charting New Paths: Increasing Patient Representation in Genitourinary Malignancy Trials
(UroToday.com) The 2024 GU ASCO annual meeting featured a Keynote Lecture by Dr. Cheryl Lee discussing increasing patient representation in genitourinary malignancy trials. Dr. Lee started her presentation by noting that there are many benefits of clinical trial participation, including that (i) cancers included in clinical trials have the greatest advances in treatment and survival, (ii) trials provide access to innovative therapies that are otherwise unavailable, and (iii) trial participants have better clinical outcomes compared to non-participants. However, there are barriers to clinical trial participation, such as trust in medical research, complexity of participation, and social determinants.
ASCO GU 2024: A New Era in the Perioperative Management of Muscle Invasive Bladder Cancer
(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma oral abstract session. Dr. Max Kates delivered the discussant for the preceding two abstract presentations:
- AMBASSADOR Alliance A031501: Phase III randomized adjuvant study of pembrolizumab in muscle-invasive and locally advanced urothelial carcinoma versus observation
- Predicting clinical outcomes in the S1314-COXEN trial using a multimodal deep learning model integrating histopathology, cell types, and gene expression
He noted that these two studies have the potential to act as ‘disrupters’ to the current muscle invasive bladder cancer (MIBC) treatment paradigm. The AI model developed by Dr. Faltas’ lab improves the prediction of treatment response to neoadjuvant chemotherapy and may allow for better selection and more widespread adoption of bladder preservation for complete responders. Dr. Apolo’s study of adjuvant pembrolizumab (AMBASSADOR) is the 2nd adjuvant immune checkpoint inhibitor therapy trial to demonstrate a disease-free survival benefit. This has important implications for adopting a multimodal, multidisciplinary approach for patients with locally advanced bladder cancer.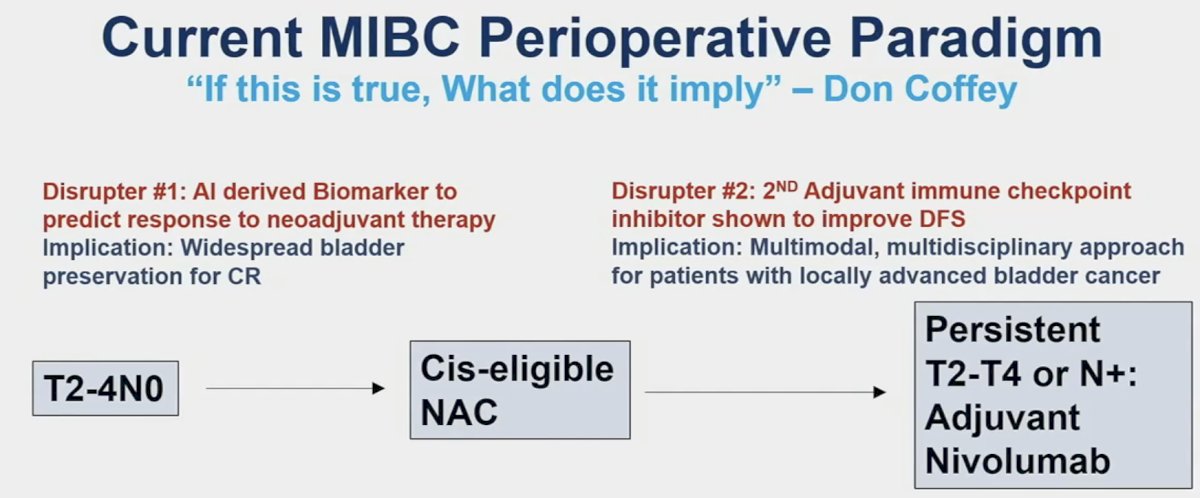
These studies also fit well with the current paradigm shift/evolution that we are witnessing in the MIBC space, whereby we are seeing an increased adoption of bladder preservation across the disease continuum, both for high-risk NMIBC and MIBC.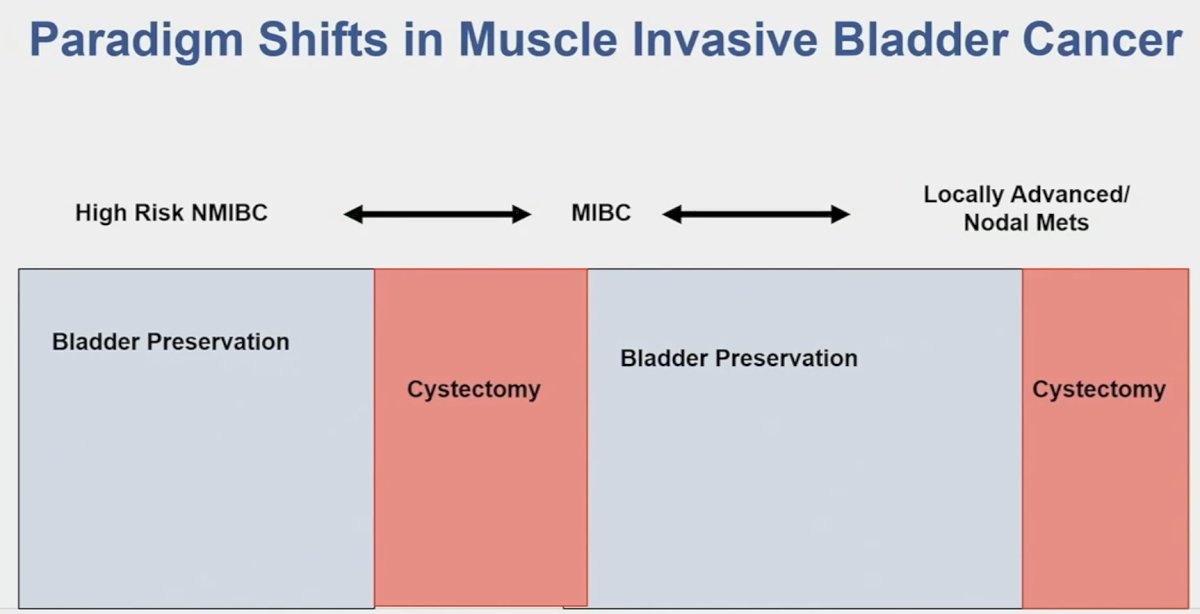
In the COXEN trial, the cohort/data of which was used to derive the AI model by Faltas et al., there was 30.8% complete response rate with neoadjuvant chemotherapy. Importantly, a pathologic complete response was a highly prognostic surrogate endpoint, as seen below, whereby CR patients had excellent long-term survival outcomes.1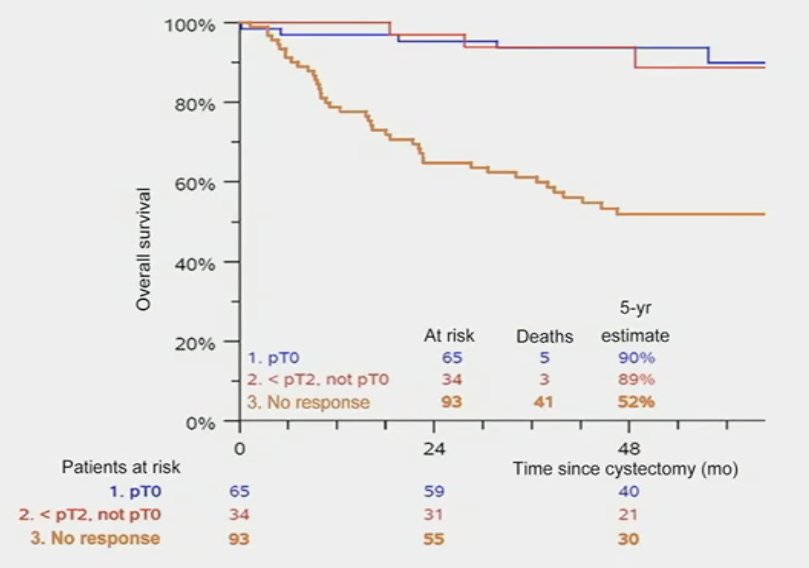
In other words, if we can reliably predict CR, then we can preserve bladders. This has been tested in several studies, including the RETAIN study, whereby patients receiving neoadjuvant ddMVAC with evidence of a complete clinical response and were mutation positive for ATM, RB1, FANCC, or ERCC2 underwent active surveillance (n=26). Of these 26 patients, 69% had disease recurrence on surveillance and 8 underwent a cystectomy. To date, 12 (46%) are metastasis-free with an intact bladder and free from bladder radiation.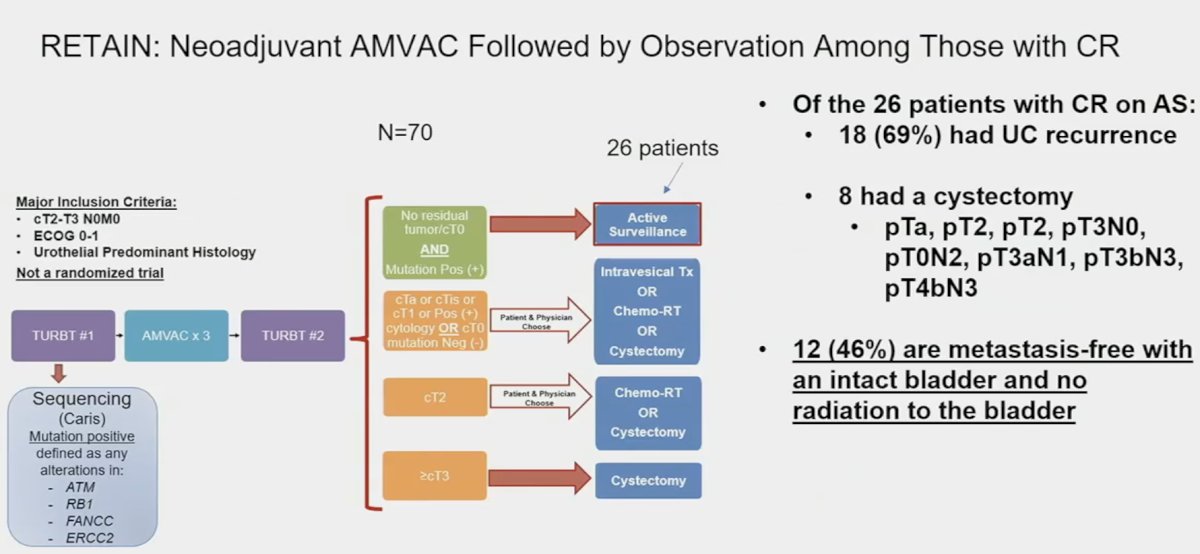
Another phase 2 trial of nivolumab + gemcitabine/cisplatin similarly evaluated outcomes among pathologic complete responders but failed to demonstrate added value to genomic testing.2 As such, additional information and tools to inform potential bladder sparing treatment decisions are sorely needed. This is where Dr. Faltas’ AI model comes into play.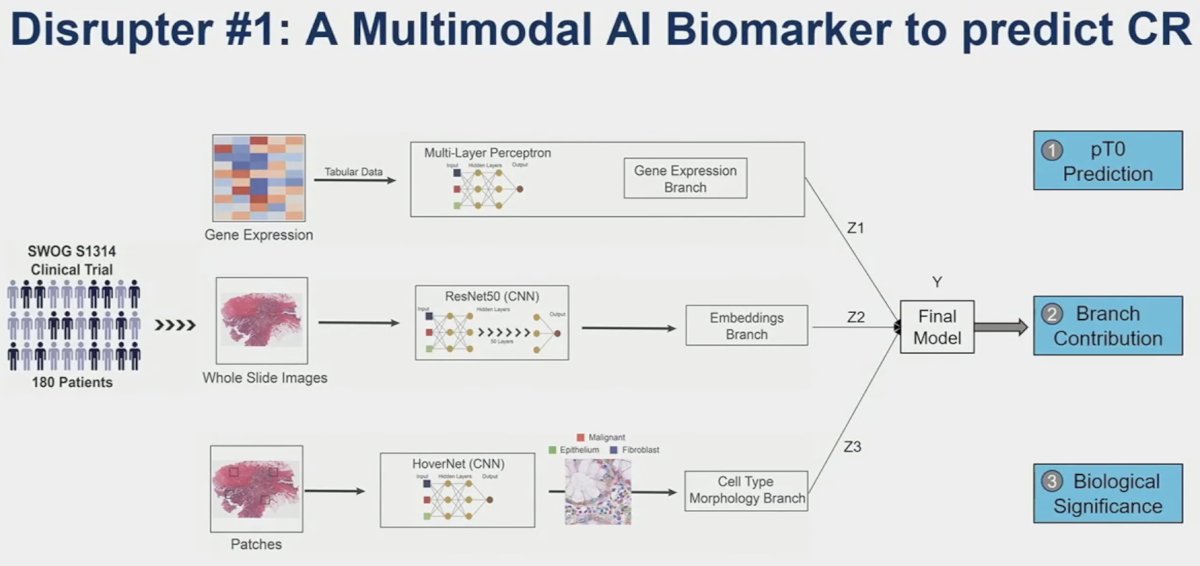
They demonstrated that this multimodal model, combining gene expression patterns, whole slide images, and cell type/morphology patches is able to predict pCR better than any single component alone. This is a valuable tool that can aid clinicians to better predict those with a CR who can retain their bladder.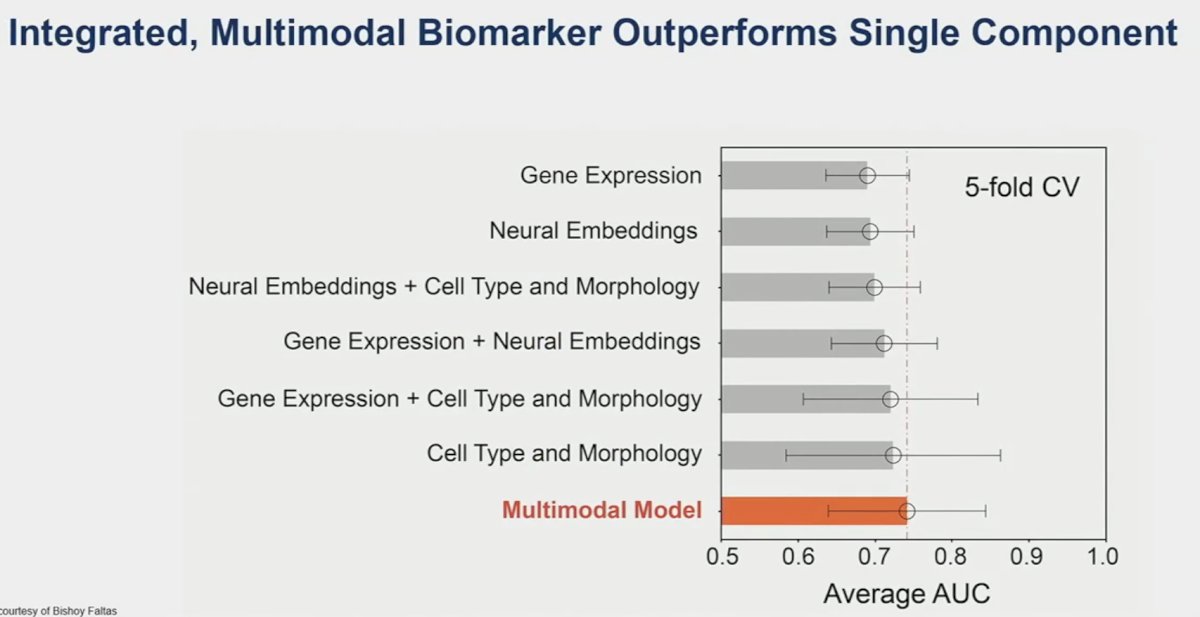
Next, Dr. Kates shifted gears to discuss ‘disrupter #2’: the AMBASSADOR trial. Previous trials in this space have included IMvigor-010 (atezolizumab) and CheckMate-274 (nivolumab).3,4 IMvigor-010 was a negative trial, failing to demonstrate a DFS benefit for atezolizumab in the adjuvant setting. Conversely, CheckMate-274 demonstrated a significant DFS benefit and is now FDA approved for this indication. Now, we have AMBASSADOR as a ‘tiebreaker’ trial, demonstrating a significant DFS benefit. Notably, this was a more heavily pre-treated cohort compared to the previous two trials, with 64% of patients receiving neoadjuvant chemotherapy (64% versus 43-48%). We also note that the median DFS in the pembrolizumab arm (29 months) was significantly longer compared to those observed in the other two active treatment arms (~20 months).
Unanswered question for adjuvant therapy in MIBC remain:
- Do all patients with high-risk features need adjuvant therapy?
- Is one year of pembrolizumab (18 cycles) or nivolumab (24 cycles) necessary for all patients?
- Is pembrolizumab monotherapy the future or is
enfortumab vedotin + pembrolizumab inevitable?
- Currently, there are two trials EV-303 and EV-304 that are evaluating the combination of enfortumab vedotin + pembrolizumab in the MIBC perioperative setting
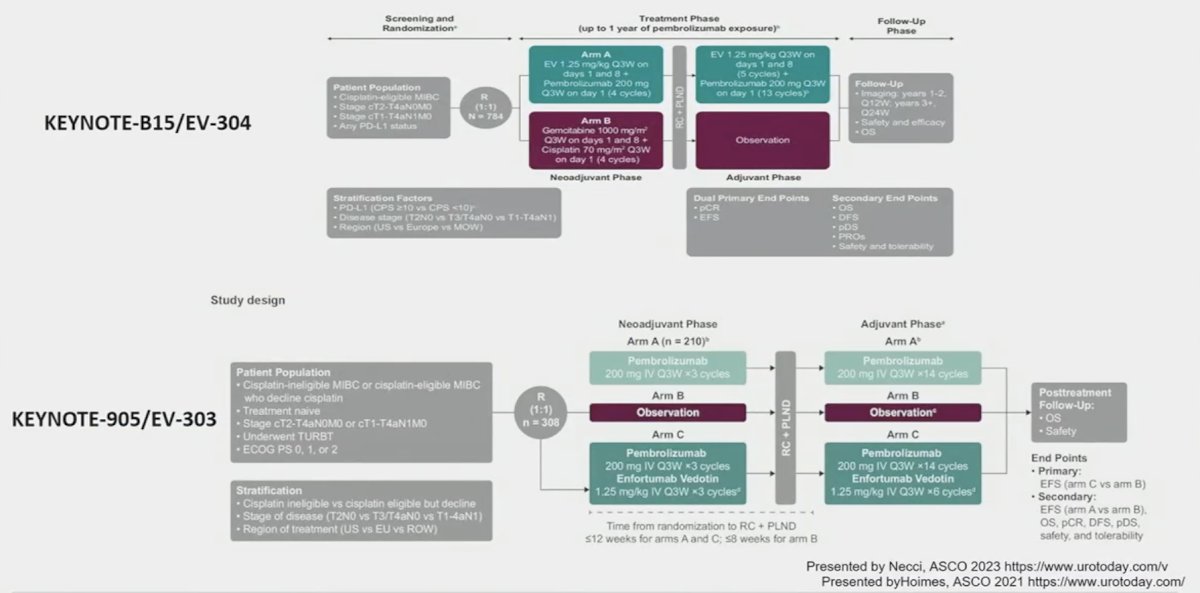
ctDNA may hold promise in this regard with secondary analysis of IMvigor-010 demonstrating that ctDNA holds value as a predictive biomarker to help determine which patients benefit from adjuvant atezolizumab therapy. This evidence is a key rationale for the A032103 (MODERN) trial that is evaluating adjuvant therapy treatment combinations (nivolumab, nivolumab + relatlimab, or surveillance) based on ctDNA status.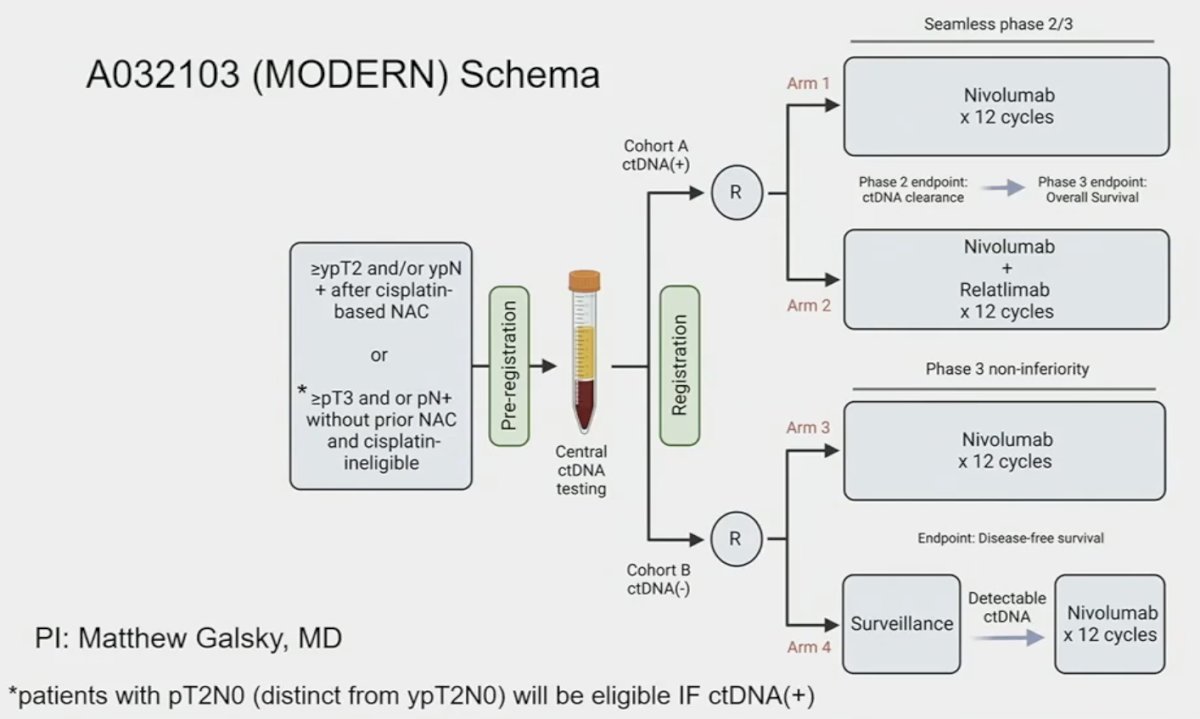
To conclude, Dr. Kates shared his vision of the future paradigm in cis-eligible patients, based on the current evidence and where he sees the field evolving: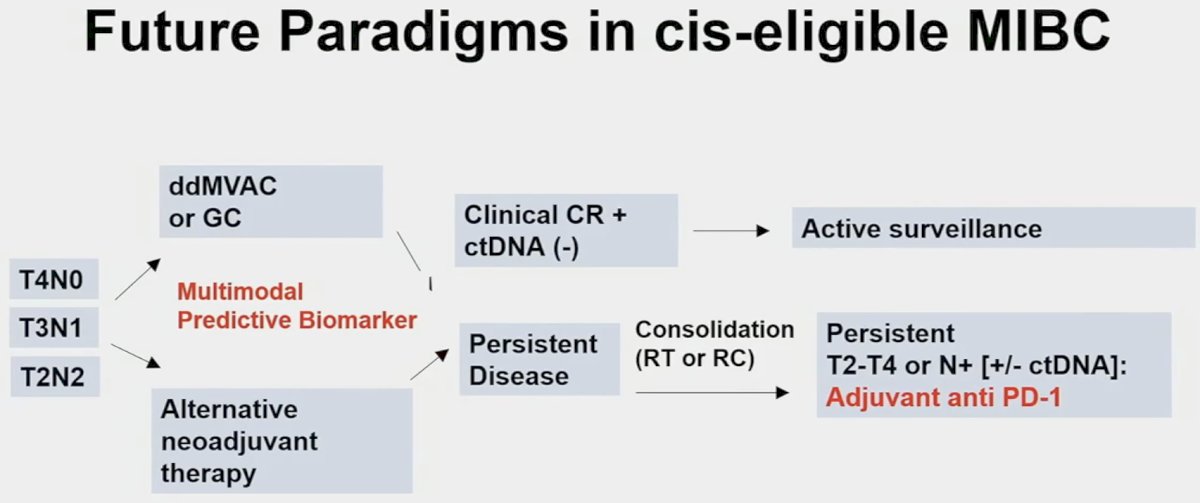
Presented by: Max R. Kates, MD, Associate Professor of Urology and Oncology, Brady Urological Institute, Johns Hopkins Medical Center, Baltimore, MD
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:- Flaig TW, Tangen CM, Daneshmand S, et al. Long-term Outcomes from a Phase 2 Study of Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer (SWOG S1314; NCT02177695). Eur Urol. 2023;84(3):341-347.
- Galsky MD, Daneshmand S, Izadmehr S, et al. Gemcitabine and cisplatin plus nivolumab as organ-sparing treatment for muscle-invasive bladder cancer: a phase 2 trial. Nat Med. 2023;29:2825-2834.
- Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2021 Apr;22(4):525-537.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.
ASCO GU 2024: Drug Sequencing, Pairing, Switching, and the Role of Checkpoint Re-Challenging
(UroToday.com) The 2024 GU ASCO annual meeting featured a session on the role of immunotherapy in advanced urothelial carcinoma and a presentation by Dr. Peter O'Donnell discussing drug sequencing, pairing, switching, and the role of checkpoint re-challenging. Dr. O’Donnell started his presentation highlighting three patient scenarios to consider:
ASCO GU 2024: Drug Shortages: Why Are They a Recurrent Issue and What Can Regulatory Do to Prevent Them?
(UroToday.com) The 2024 GU ASCO annual meeting featured a session on the shortage of drugs for urothelial carcinoma and a presentation by Dr. Chana Weinstock discussing drug shortages, why there are recurrent issues, and what regulations can do to prevent them. Dr. Weinstock started by highlighting that drug shortages are often visible to the public, such as the press on Twitter and the New York Times. So, with regard to drug shortages, how did we get here? What is the FDA doing about this? What are some long-term solutions?
ASCO GU 2024: Lessons Learned: What Can Other Disease Sites Teach Us About Practical Implications of Checkpoint Therapy in Urothelial Carcinoma?
(UroToday.com) The 2024 GU ASCO annual meeting featured a session on the role of immunotherapy in advanced urothelial carcinoma and a presentation by Dr. Monty Pal discussing what we can learn from other disease sites regarding the implications of checkpoint therapy in urothelial carcinoma. Dr. Pal emphasized that the best thing to do when handling tough topics is to use the “phone a friend” approach, specifically when there are so many medical oncology experts across the globe:
ASCO GU 2024: Surgical Therapeutic Role for Node-Positive Disease: Does It Make a Difference?
(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a session on optimizing treatment strategies for patients with positive pelvic and/or retroperitoneal lymph nodes. Dr. Robert Svatek discussed whether surgical therapy has a role for the management of urologic oncology patients with node-positive disease.
ASCO GU 2024: Genetic Testing in RCC: Who, When, and How?
(UroToday.com) The 2024 GU ASCO annual meeting featured a session on the management of renal cell carcinoma with sarcomatoid features or variant histologies and a presentation by Dr. Ramaprasad Srinivasan discussing who, when, and how to perform genetic testing. Dr. Srinivasan started his presentation by emphasizing that genetic testing in kidney cancer is starting to come of age. This is likely secondary to a heightened understanding of the genetic basis of various kidney cancer subtypes and that there are now 20 known genes to be associated with kidney cancer. However, based on the known drivers of kidney cancer, it is critical to develop individualized treatment strategies and to utilize the easy access to genetic testing.
ASCO GU 2024: Management of Non-Clear Cell Renal Cell Carcinoma
(UroToday.com) The 2024 GU ASCO annual meeting featured a session on the management of renal cell carcinoma with sarcomatoid features or variant histologies and a presentation by Dr. Thomas Powles discussing the management of non-clear cell renal cell carcinoma. Dr. Powles started his presentation by discussing the ESPN trial,1 which tested sunitinib versus everolimus among 68 patients with non-clear cell RCC, noting an overall response rate of 9% versus 3%, median progression free survival of 6.1 versus 4.1 months, and overall survival of 16.2 versus 14.9 months:
ASCO GU 2024: The Emergent Role of Theranostics in Renal Cell Carcinoma
(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a session addressing the incorporation of radiation therapy, theranostics, and biomarkers into the management of renal cell carcinoma (RCC). Dr. Brian Shuch discussed the emergent role of theranostics in RCC.
ASCO GU 2024: Harnessing the Power: Biologic Basis of Pairing and Sequencing Checkpoints with Other Therapy
(UroToday.com) The 2024 GU ASCO annual meeting featured a session on the role of immunotherapy in advanced urothelial carcinoma and a presentation by Dr. William Kim discussing the biologic basis of pairing and sequencing checkpoints with other therapies. Dr. Kim started by asking the question “What do we know about the bladder cancer tumor microenvironment?” Generally, urothelial carcinoma has a high tumor mutational burden, and antigen driven immune responses likely account for immune checkpoint inhibitor responses:
ASCO GU 2024: The NEPI Trial: A Randomized Phase I/II Study of Neoadjuvant Treatment with 177-Lutetium-PSMA-617 with or without Ipilimumab in Patients with Very High-Risk Prostate Cancer Who Are Candidates for Radical Prostatectomy
(UroToday.com) The 2024 GU ASCO annual meeting included a prostate cancer session featuring trials in progress and a presentation by Dr. Ulrich Krafft discussing the trial design of the NEPI trial, a randomized phase I/II study of neoadjuvant treatment with 177-Lutetium-PSMA-617 (LuPSMA) with or without ipilimumab in patients with very high-risk prostate cancer who are candidates for radical prostatectomy. High-risk prostate cancer accounts for approximately 15% of newly diagnosed prostate cancers, and for these patients with high-risk, locally advanced prostate cancer, prostatectomy alone may be insufficient therapy. Indeed, cure rates from radical prostatectomy alone are less than 25%.
ASCO GU 2024: A Randomized, Non-Comparative, Phase II Multicenter Trial of Short-Term Darolutamide Concomitant to Radiation Therapy for Patients with Unfavorable Intermediate-Risk Prostate Cancer: DARIUS (AFU-GETUG P15)
(UroToday.com) The 2024 GU ASCO annual meeting included a prostate cancer session featuring trials in progress and a presentation by Dr. Guilhem Roubaud discussing the trial design of DARIUS (AFU-GETUG P15), a randomized, non-comparative, phase II multicenter trial of short-term darolutamide concomitant to radiation therapy for patients with unfavorable intermediate-risk prostate cancer. ADT with external beam radiation therapy is a standard of care for patients with unfavorable intermediate-risk prostate cancer. However, ADT generates side effects with a 30% decrease of quality of life. Enzalutamide and darolutamide combined with ADT offer an overall survival benefit in advanced prostate cancer, and without ADT, enzalutamide avoids most of the deleterious effects associated with ADT.


