(UroToday.com) The Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2024 Annual Meeting held in Toronto, ON between June 8th and June 11th, 2024 was host to a prostate-specific membrane antigen (PSMA) radioligand therapy (RLT) tumor board session. Dr. Louise Emmett discussed the rationale for and against combining RLT with other agents in treating prostate cancer patients.
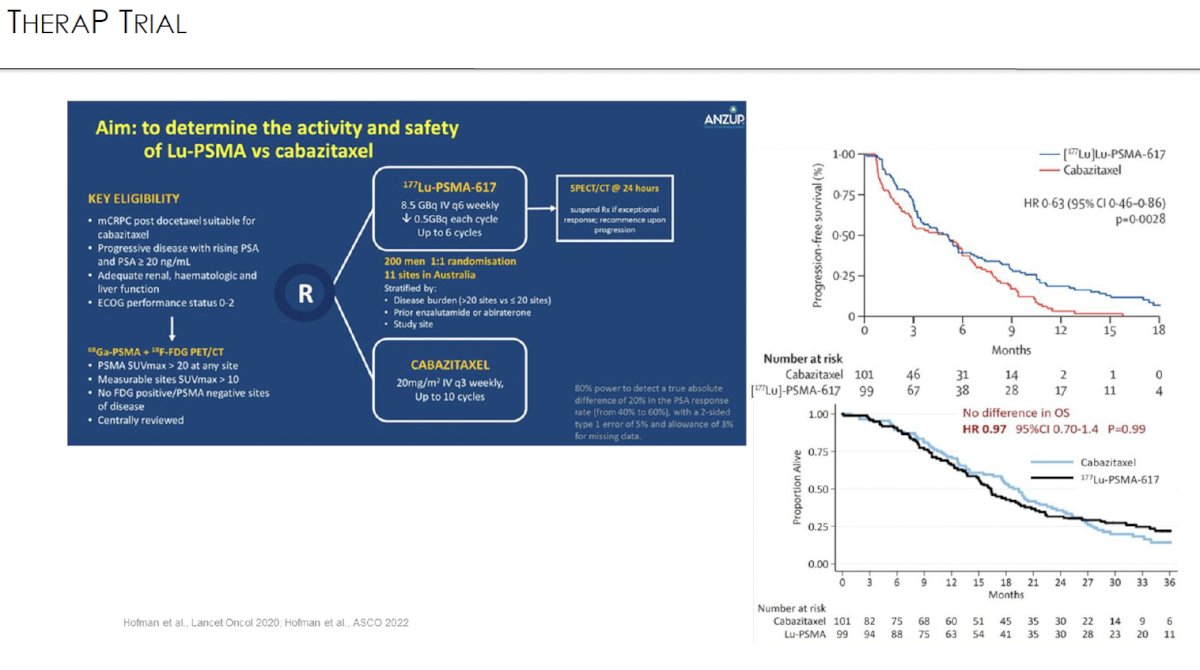
To date, there have been two major trials of 177Lu-PSMA-617 for metastatic castrate-resistant prostate cancer (mCRPC): VISION and TheraP.1,2
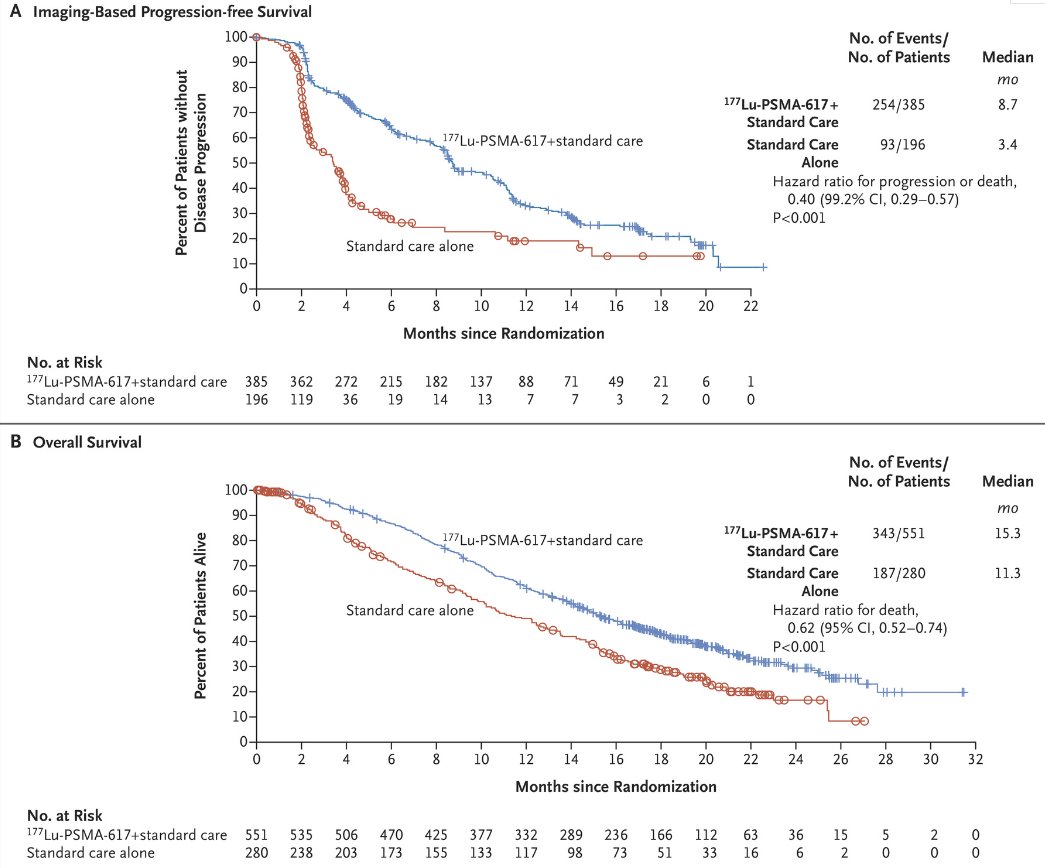
VISION is an international, open-label, phase 3 trial that evaluated 177Lu-PSMA-617 in mCRPC patients previously treated with an androgen receptor pathway inhibitor (ARPI) and 1-2 taxane regimens and who had PSMA-positive 68Ga-PSMA-PET/CT scans. Between June 2018 and October 2019, 831 patients were randomly assigned in a 2:1 ratio to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks for four to six cycles) plus protocol-permitted standard care or standard care alone. At a median follow-up of 20.9 months, 177Lu-PSMA-617 plus standard care significantly prolonged, as compared with standard of care, both radiographic progression-free survival (rPFS; median: 8.7 versus 3.4 months; HR: 0.40, p<0.001) and overall survival (median: 15.3 versus 11.3 months; HR: 0.62; 95% CI: 0.52 to 0.74, p<0.001).
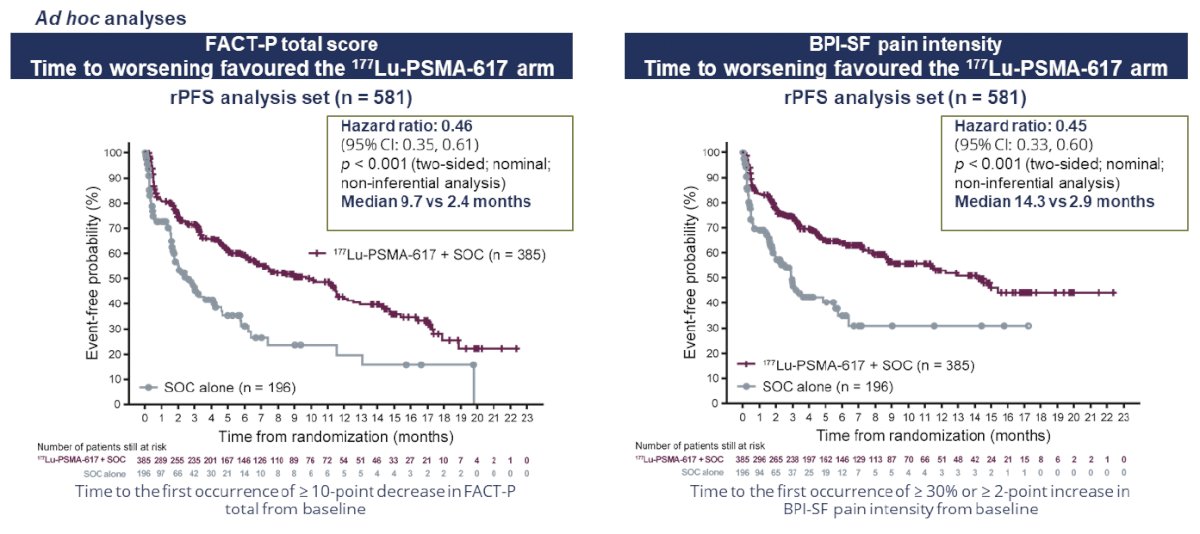
Patient reported outcomes as evaluated by the FACT-P and Brief Pain Inventory (BPI) scores favored the 177Lu-PSMA-617 arm with delays in time to worsening of 7.3 and 11.4 months, respectively.
While a higher rate of high-grade (grade 3-5) treatment-emergent adverse events was observed with 177Lu-PSMA-617 (28.4% versus 3.9%) at the time of initial reporting, overall therapy was well tolerated. It bears note that treatment exposure was more than three times longer in the 177Lu-PSMA-617 group than in the control group.

TheraP was an open label, phase II trial of 200 mCRPC men who were randomized to either 177Lu-PSMA-617 or cabazitaxel. To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (≥1 site with SUVmax ≥20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. 200 patients were randomized 1:1 to 177Lu-PSMA-617 6-8 GBq every 6 weeks for up to 6 cycles of therapy or cabazitaxel 20 mg/m2 every three weeks for up to 10 cycles.
The most recent update of TheraP was presented at ASCO 2022 after a median follow-up of 36 months. Progression-free survival favored LuPSMA (HR: 0.62, 95% CI: 0.45 – 0.85). There was however no significant difference in overall survival between the two arms (restricted mean survival time: 19.1 months in 177Lu-PSMA-617 arm versus 19.6 months in cabazitaxel arm, 95% CI for difference: -3.7 - +2.7).3
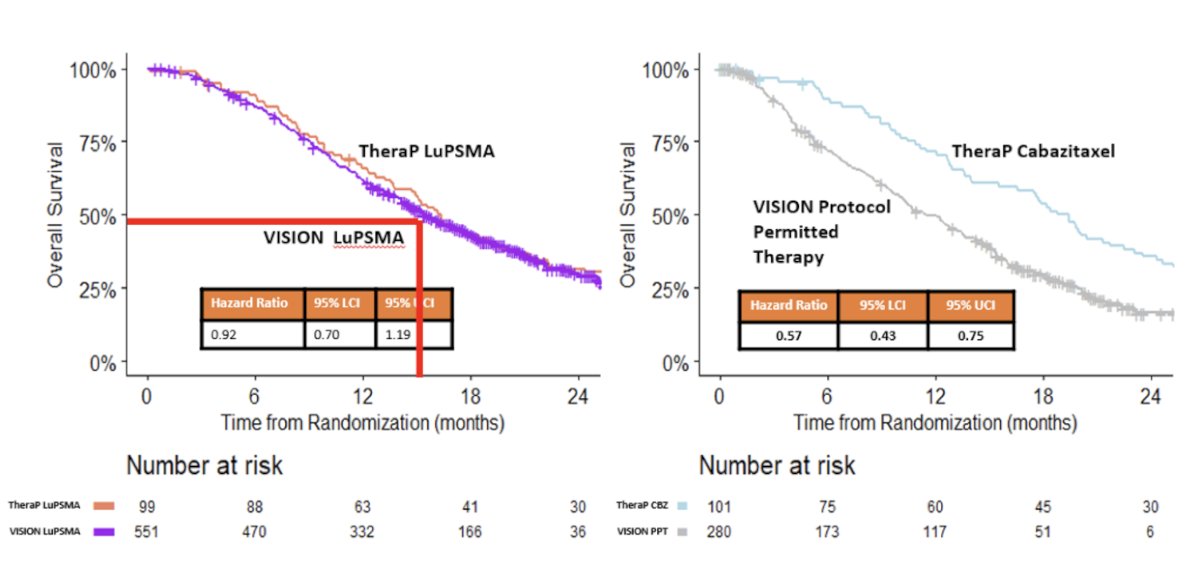
An exploratory analysis of the varying effects of 177Lu-PSMA-617 on overall survival in TheraP versus VISION was presented at ASCO 2023 – why were the hazard ratios (HRs) for 177Lu-PSMA-617 significantly different in these two trials (VISION: 0.62 versus TheraP: 0.97). First, it appears that the choice of comparator arm makes a significant difference. Patients in the cabazitaxel comparator arm of TheraP had a significantly longer overall survival compared to those in the standard of care arm in the VISION trial (HR: 0.57, 95% CI: 0.43 – 0.75). Overall survival was similar in the 177Lu-PSMA-617 groups of VISION and TheraP (HR: 0.92, 95% CI: 0.70 – 1.19). No significant differences in overall survival between the 177Lu-PSMA-617 and cabazitaxel groups of TheraP were apparent using counterfactual analyses assuming crossovers had not occurred. As such, the investigators concluded that the difference in observed effects of 177Lu-PSMA-617 on overall survival in TheraP and VISION are better explained by the use of different comparator treatments in their control groups than by crossovers in TheraP from cabazitaxel to 177Lu-PSMA-617, or 177Lu-PSMA-617 to cabazitaxel.
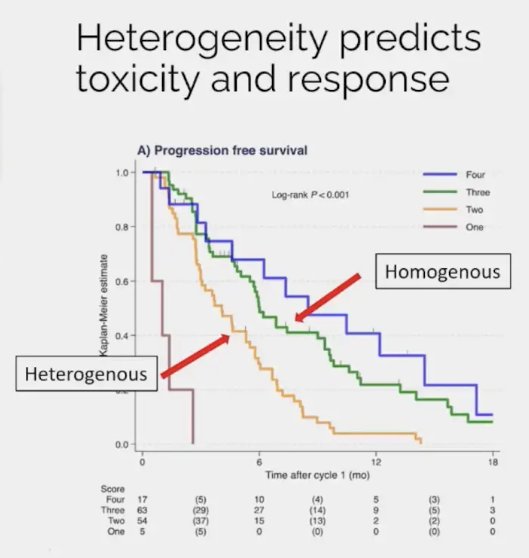
A rationale for considering Lu-PSMA combination therapies is that patients with evidence of disease visual heterogeneity on 68Ga-PSMA-11 PET/CT have worse disease outcomes with radioligand therapy. It is important to consider the likelihood of treatment benefit in candidate patients. Recently, Dr. Emmet’s group developed a 4-category score incorporating both heterogeneity and intensity of tumors (HIT) using a combination of heterogeneity and intensity:3
- Category 1: SUVmax <15
- Category 2: SUVmax 15-79 with heterogeneous intensity
- Category 3: SUVmax 15-79 with homogenous intensity
- Category 4: SUVmax ≥80
As demonstrated in the Kaplan Meier curve below, patients in the higher category groups had superior outcomes with Lu-PSMA therapy. The median PSA progression-free survivals were:
- Category 1: 1 month
- Category 2: 4.1 month
- Category 3: 6.0 month
- Category 4: 8.5 month
As such, patients with heterogenous PSMA expression may be ideal candidates for combination approaches, that would ideally achieve all of the following:
- Combining PSMA-targeting treatment with an agent that has a complementary effect that targets cells that do not express PSMA.
- Combine PSMA-targeting treatment with an agent likely to enhance PSMA expression.
- Combine PSMA-targeting treatment with an agent likely to enhance radiation sensitivity.
- Not significantly increasing toxicity

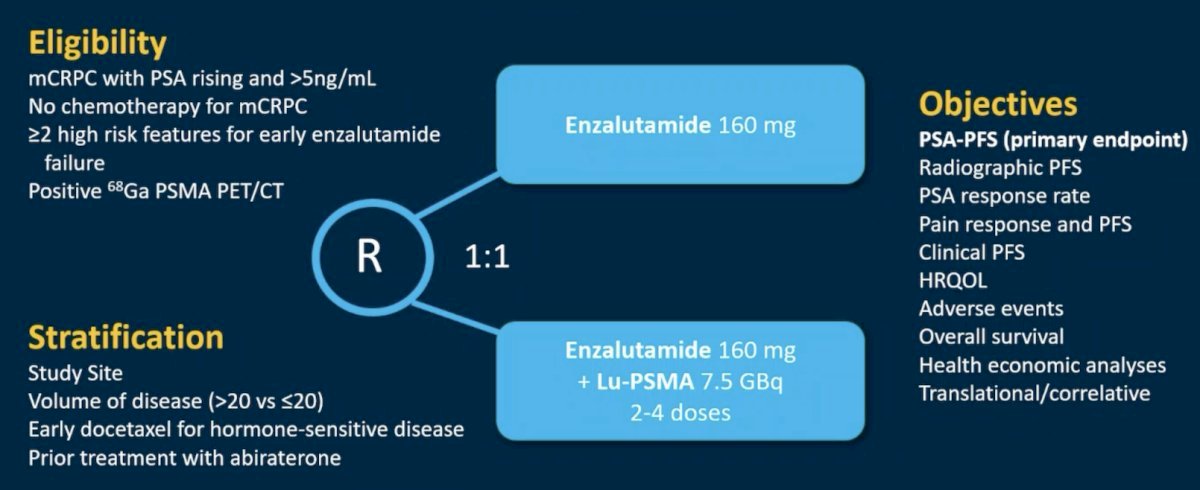
One example of a RLT combinatory approach that allows for the targeting of cells without PSMA expression is the combination of 177Lu-PSMA-617 + enzalutamide. There is a close relationship between the androgen and PSMA receptors, with upregulation of PSMA expression in response to androgen blockade observed in mCRPC. This increased PSMA expression is associated with poor survival in CRPC patients receiving ARPIs. Increased PSMA expression increases dsDNA breaks and cell death with Lu-PSMA therapy, increasing the depth of treatment response. This reduces/eliminates the high PSMA expressing clonal population, leaving a low PSMA expression clonal population more likely to respond to ARPIs. The ENZA-p trial investigators hypothesized that using both treatments together may lead to potentially deeper and longer patient responses, compared to ARPI monotherapy.

ENZA-p (ANZUP 1901) is an open label, randomized phase 2 trial across 15 centers in Australia of 162 mCRPC patients who had not previously received a taxane or an ARPI in the mCRPC setting, had 68Ga-PSMA-PET/CT-positive disease, and ≥2 risk factors for early progression on enzalutamide. These patients underwent 1:1 randomization to enzalutamide +/- 177Lu-PSMA-617.
Of 220 screened patients, 160 met the eligibility criteria and were randomized (enzalutamide + Lu-PSMA, n=83; enzalutamide, n=79). The PSMA PET imaging screen failure rate was 18%. In the combination arm, 81% of patients received all 4 doses of Lu-PSMA. The median follow-up was 20 months.
This trial met its primary endpoint, with the addition of 177Lu-PSMA-617 to enzalutamide improving PSA progression-free survival from 7.8 to 13 months (HR: 0.43, 95% CI: 0.29 – 0.63, p<0.001). rPFS (data maturity: 60%) also favored the combination arm (median: 16 versus 12 months; HR: 0.67, 95% CI: 0.44 – 1.01).

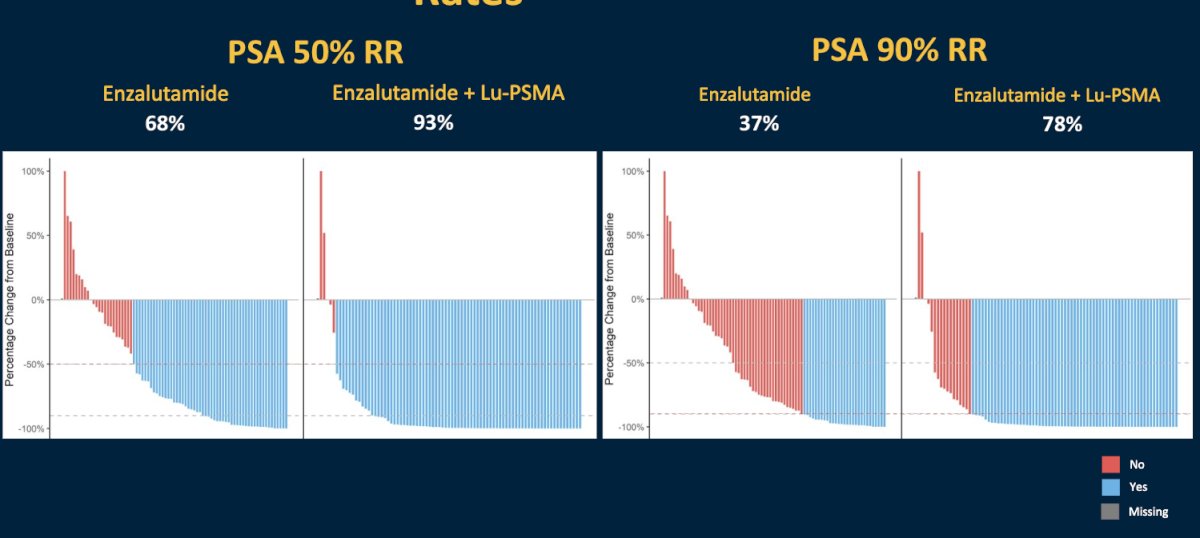
A PSA50 response was observed in 93% of patients in the Lu-PSMA + enzalutamide arm, compared to 68% of enzalutamide-treated patients. The corresponding PSA90 responses were 78% and 37%, respectively.4
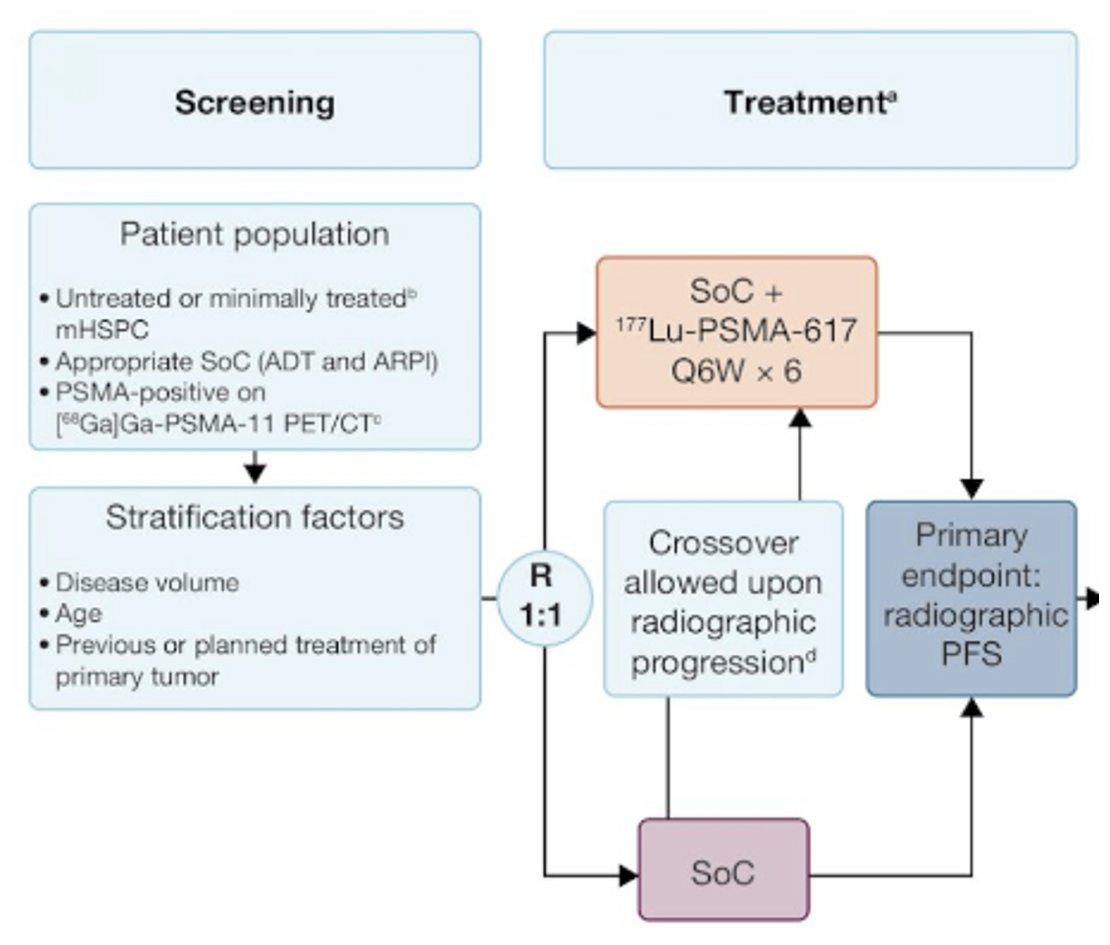
In the metastatic hormone-sensitive setting, the combination of Lu-PSMA and an ARPI is being evaluated in PSMAddition, summarized below. This is a phase 3 trial of 177Lu-PSMA-617 + standard of care versus standard of care alone in patients with mHSPC (n=1,226). The study design is illustrated below. The primary study endpoint is rPFS, with crossover permitted at radiographic progression. Overall survival is a key secondary endpoint. Recruitment for this trial was completed in 2023, with an estimated study completion date of February 2026.
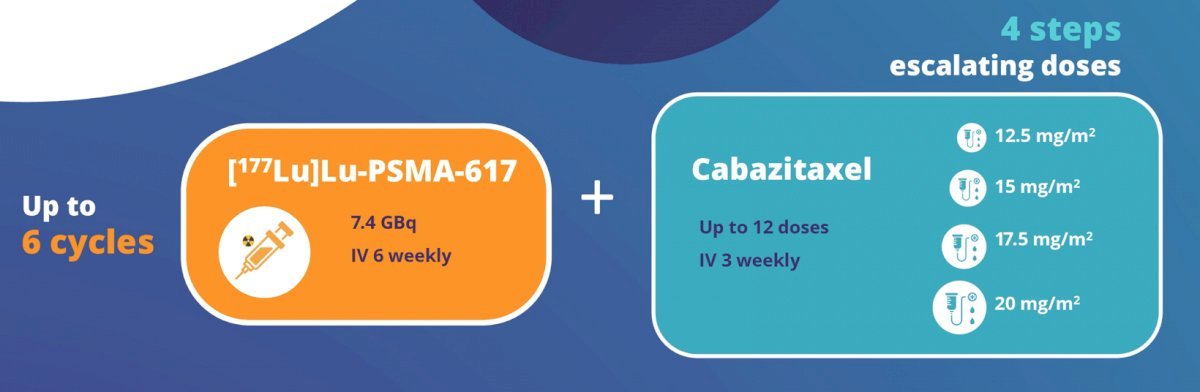
The combination of 177Lu-PSMA-617 and cabazitaxel has been evaluated in the phase I/II LuCAB trial. Cabazitaxel has radiosensitizing properties that may enhance the cytotoxic effect of 177Lu-PSMA-617, while also treating any PSMA-negative disease. Additionally, systemic chemotherapy may effectively treat micrometastatic disease that may not be targeted by the beta emitter, 177Lu-PSMA-617. LuCAB is a single arm phase I/II trial is evaluating the combination of 177Lu-PSMA-617 plus cabazitaxel in mCRPC patients with disease progression following prior ARPI and docetaxel exposure and evidence of PSMA-positive disease on PSMA-PET/CT (SUVmax ≥15). The target sample size is 32 – 38 patients. Up to 6 doses of 177Lu-PSMA-617 (7.4 GBq) will be administered intravenously every 6 weeks. Cabazitaxel will be given concurrently (dose range 12.5mg/m2 - 20mg/m2), on Day 2 and Day 23 of each 6-week cycle:
The primary objective is to establish the maximum tolerated dose of cabazitaxel and [177Lu-PSMA-617. Secondary objectives include:
- Measuring the frequency and severity of adverse events
- Assessment of efficacy through PSA 50% response rate
- Radiographic and PSA progression-free survival
- Overall survival
- Objective tumor response rate
- Evaluation of pain and health-related quality of life over the first 12 months
As of January 2023, five patients have been enrolled in the study, with preliminary results expected in the upcoming few years.5
What about combinations to enhance PSMA expression and thus potentially improve the efficacy of radioligand therapy? A screen of 147 anticancer agents demonstrated that the topoisomerase-2 inhibitors, daunorubicin and mitoxantrone, upregulated PSMA protein expression in castration-resistant LNCaP95 cells. Additionally, ionizing radiation also upregulated PSMA protein expression in a dose-dependent fashion.6

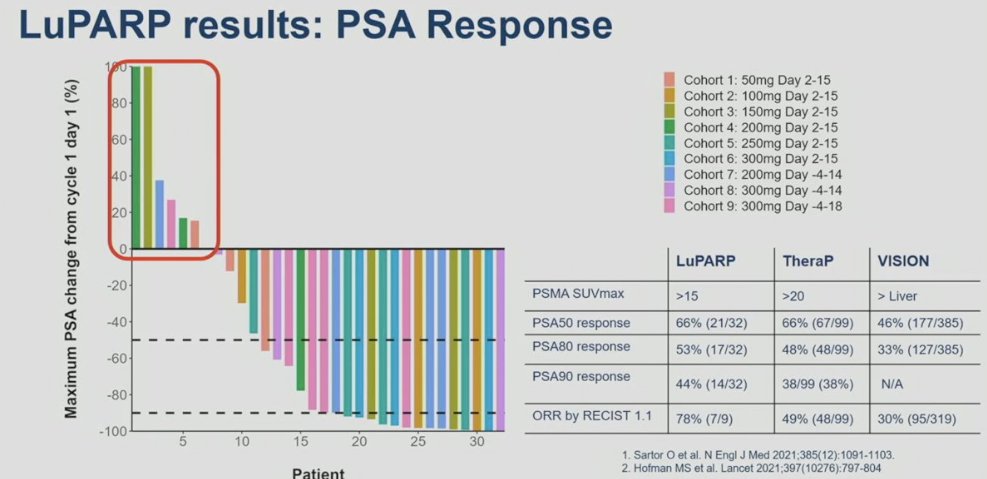
What about combining PSMA targeting treatment with an agent likely to enhance radiation sensitivity/cell death? 177Lu-PSMA-617 delivers significant Beta radiation to PSMA-expressing tumors, primarily causing single strand DNA breaks, which are typically repaired by PARP-dependent pathways. Blocking PARP could result in the conversion of DNA single strand breaks to lethal double strand breaks via replication fork collapse. In the LuPARP trial, the investigators hypothesized that the addition of olaparib could promote the radiosensitization of 177Lu-PSMA-617, resulting in DNA damage intensification, and thus improved efficacy.
In LuPARP, 48 patients with mCRPC in the post-ARPI/docetaxel setting and PSMA expression (SUVmax 15 at a site of disease, and SUVmax ≥ 10 at other measurable sites) without discordant FDG positive/PSMA negative disease, received the combination of 177Lu-PSMA-617 + olaparib in in two stages: dose-escalation (standard 3+3 design) and dose-expansion at the recommended phase 2 dose (RP2D). In this phase 1 trial, 177Lu-PSMA-617 was administered at a dose of 7.4 GBq 6 weekly for 6 cycles. Olaparib was concurrently administered at a dose of 50 to 300 mg twice daily on days 2 to 15, -4 to 14, or -4 to 18 of each 6-week cycle. No dose-limiting toxicities were reported across the dose levels. There were no grade 4 adverse events. One treatment-related serious adverse event occurred (febrile neutropenia). From an efficacy standpoint, the PSA50 and PSA90 response rates in the overall cohort were 66% and 44%, respectively. The objective response rate by RECIST v1.1 criteria was 78%. How do these results compare to those from TheraP and VISION? The PSA50 responses from this trial were identical to those from TheraP (66%) and higher than that in VISION (46%). The PSA90 response of 44% in LuPARP was slightly higher than that in TheraP (38%).7
The combination of 177Lu-PSMA-617 plus pembrolizumab is being evaluated in the phase I PRINCE trial (NCT03658447). It has been hypothesized that by potentially inducing immunogenic cell death, 177Lu-PSMA-617 may act synergistically with pembrolizumab to enhance the depth and durability of response. In this trial, mCRPC patients with high PSMA expression (SUVmax ≥ 20 in an index lesion, SUVmax > 10 for all lesions ≥ 10mm), and no FDG positive/PSMA negative lesions on paired baseline PET/CT screening, received up to 6 cycles of 177Lu-PSMA-617 (starting at 8.5 GBq, reducing by 0.5 GBq with each cycle) every 6 weeks in conjunction with 200 mg of pembrolizumab every 3 weeks for up to two years. The study schema is as follows:

There were 37 patients (73% had received prior docetaxel, and 100% had received a prior ARPI) that received a median of 5 cycles (range 2 to 6) of 177Lu-PSMA-617 and 12 doses (range 6 to 19) of pembrolizumab. The median follow up was 16 months, over which time the 50% PSA response rate was 76% and 7/10 (70%) patients with RECIST-measurable disease had a partial response. The median rPFS was 11.2 months. The median PSA-progression-free survival was 8.2 months, and the median overall survival was 17.8 months.
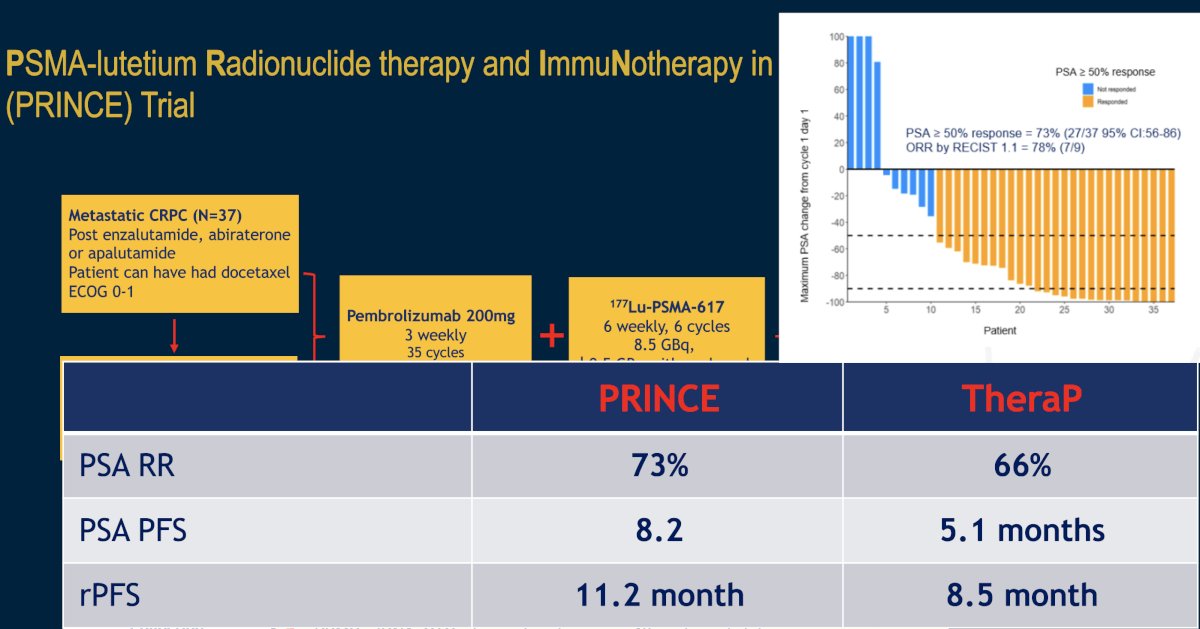
Common (≥10%) treatment-related adverse events were mainly grade 1-2, including xerostomia (78%), fatigue (43%), pruritus (27%), nausea (27%), and rash (24%). Hematologic treatment-related adverse events included grade 2-3 anemia (8%), grade 1-2 thrombocytopenia (16%), and grade 1 neutropenia (3%). Grade 3 immune-related adverse events occurred in 10 (27%) patients with no dominant manifestation. There were 5 (14%) patients who discontinued pembrolizumab due to toxicity.
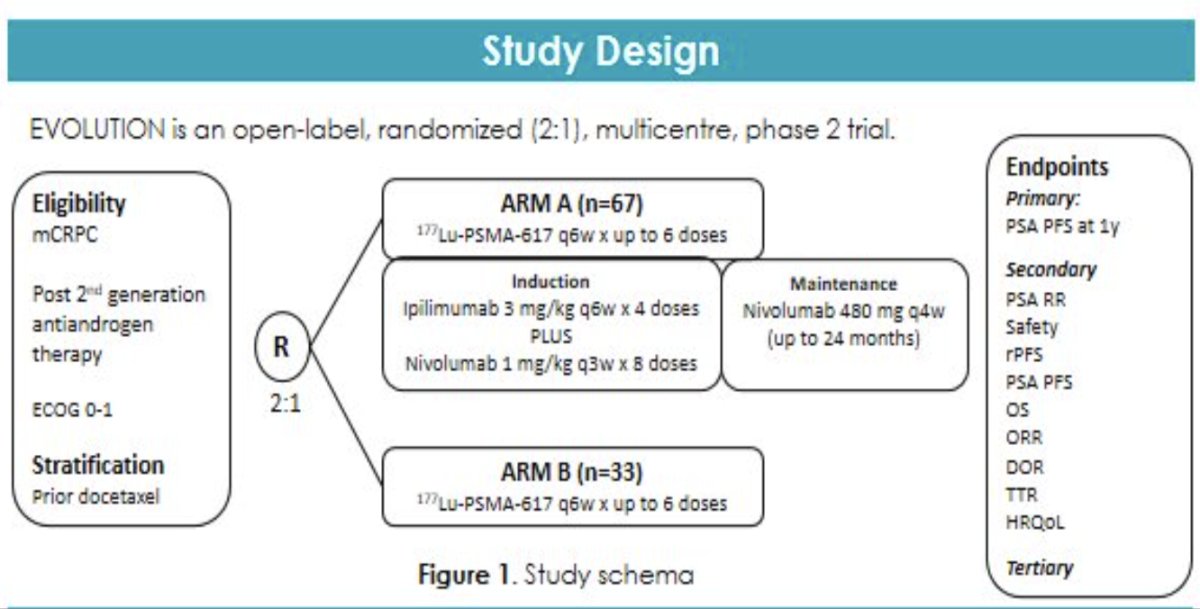
Another 177Lu-PSMA-617 + immunotherapy combination currently under investigation in the mCRPC setting is the combination of 177Lu-PSMA-617 plus ipilimumab/nivolumab, which is being evaluated in the phase II EVOLUTION trial.
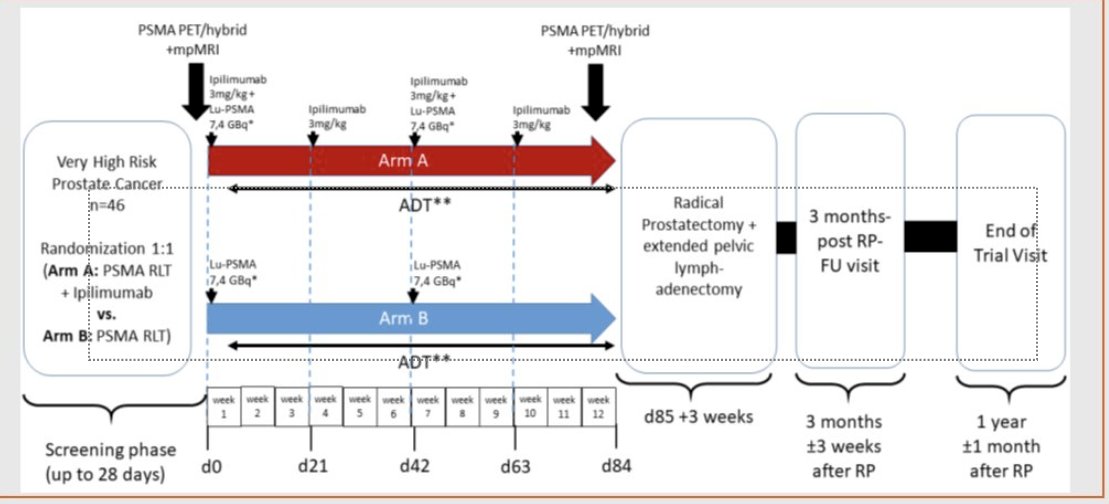
In the neoadjuvant setting, 177Lu-PSMA-617 +/- ipilimumab is being evaluated in the NEPI trial of very high-risk prostate cancer patients who are planned for a radical prostatectomy.
Dr. Emmett concluded by noting the following regarding radioligand combination therapy approaches:
- Moving radioligand treatment into an earlier (pre-chemotherapy) setting is important, as it is better tolerated than chemotherapy regimens and may potentially improve overall survival outcomes.
- Need to ensure an additive benefit when combining radioligand therapy with other agents.
- We need to define the natural place of radioligand therapy +/- combinations in the prostate cancer journey:
- Primary role or as an adjuvant treatment
Presented by: Louise Emmett, BSc(HONS), MBChB, FRACP, FAANMS, MD, The University of New South Wales (UNSW), Sydney, Australia
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1901-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Swiha M, Papa N, Sabahi Z, et al. Development of a Visually Calculated SUVmean (HIT Score) on Screening PSMA PET/CT to Predict Treatment Response to 177Lu-PSMA Therapy: Comparison with Quantitative SUVmean and Patient Outcomes. J Nucl Med. 2024.
- Emmett L, Subramaniam S, Crumbaker M, et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024:S1470-2045(24)00135-9
- Kostos LK, Buteau JP, Kong G, et al. LuCAB: A phase I/II trial evaluating cabazitaxel in combination with [177Lu]Lu-PSMA-617 in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(Suppl 6):TPS278.
- Sheehan B, Neeb A, Buroni L, et al. Prostate-Specific Membrane Antigen Expression and Response to DNA Damaging Agents in Prostate Cancer. Clin Cancer Res. 2022;28(14): 3104-15.
- Sandhu S, Joshua AM, Emmett L, et al. LuPARP: Phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41: Suppl 16.